Prosthetic implantation of the human vestibular system
- PMID: 24317220
- PMCID: PMC4369342
- DOI: 10.1097/MAO.0000000000000003
Prosthetic implantation of the human vestibular system
Abstract
Hypothesis: A functional vestibular prosthesis can be implanted in human such that electrical stimulation of each semicircular canal produces canal-specific eye movements while preserving vestibular and auditory function.
Background: A number of vestibular disorders could be treated with prosthetic stimulation of the vestibular end organs. We have previously demonstrated in rhesus monkeys that a vestibular neurostimulator, based on the Nucleus Freedom cochlear implant, can produce canal-specific electrically evoked eye movements while preserving auditory and vestibular function. An investigational device exemption has been obtained from the FDA to study the feasibility of treating uncontrolled Ménière's disease with the device.
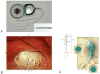
Methods: The UW/Nucleus vestibular implant was implanted in the perilymphatic space adjacent to the three semicircular canal ampullae of a human subject with uncontrolled Ménière's disease. Preoperative and postoperative vestibular and auditory function was assessed. Electrically evoked eye movements were measured at 2 time points postoperatively.
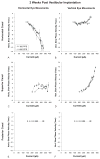
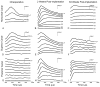
Results: Implantation of all semicircular canals was technically feasible. Horizontal canal and auditory function were largely, but not totally, lost. Electrode stimulation in 2 of 3 canals resulted in canal-appropriate eye movements. Over time, stimulation thresholds increased.
Conclusion: Prosthetic implantation of the semicircular canals in humans is technically feasible. Electrical stimulation resulted in canal-specific eye movements, although thresholds increased over time. Preservation of native auditory and vestibular function, previously observed in animals, was not demonstrated in a single subject with advanced Ménière's disease.
Figures





References
-
- Gong W, Merfeld DM. Prototype neural semicircular canal prosthesis using patterned electrical stimulation. Ann Biomed Eng. 2000;28:572–81. - PubMed
-
- Merfeld DM, Haburcakova C, Gong W, et al. Chronic vestibulo-ocular reflexes evoked by a vestibular prosthesis. IEEE Trans Biomed Eng. 2007;54:1005–15. - PubMed
-
- Phillips JO, Bierer SM, Ling L, et al. Real-time communication of head velocity and acceleration for an externally mounted vestibular prosthesis. Proceedings of the IEEE Engineering in Medicine and Biology Society. 2011;2011:3537–41. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

