Quantifying cell-generated mechanical forces within living embryonic tissues
- PMID: 24317254
- PMCID: PMC3939080
- DOI: 10.1038/nmeth.2761
Quantifying cell-generated mechanical forces within living embryonic tissues
Erratum in
- Nat Methods. 2014 Mar;11(3):349
Abstract
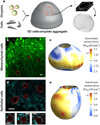
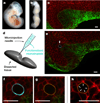
Cell-generated mechanical forces play a critical role during tissue morphogenesis and organ formation in the embryo. Little is known about how these forces shape embryonic organs, mainly because it has not been possible to measure cellular forces within developing three-dimensional (3D) tissues in vivo. We present a method to quantify cell-generated mechanical stresses exerted locally within living embryonic tissues, using fluorescent, cell-sized oil microdroplets with defined mechanical properties and coated with adhesion receptor ligands. After a droplet is introduced between cells in a tissue, local stresses are determined from droplet shape deformations, measured using fluorescence microscopy and computerized image analysis. Using this method, we quantified the anisotropic stresses generated by mammary epithelial cells cultured within 3D aggregates, and we confirmed that these stresses (3.4 nN μm(-2)) are dependent on myosin II activity and are more than twofold larger than stresses generated by cells of embryonic tooth mesenchyme, either within cultured aggregates or in developing whole mouse mandibles.
Figures


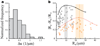
References
-
- Thompson DW. On Growth and Form. Dover reprint of 1942, 2nd ed. (1st ed.) 1917
-
- Butler LC, et al. Cell shape changes indicate a role for extrinsic tensile forces in Drosophila germ-band extension. Nat. Cell Biol. 2009;11:859U181. - PubMed
Methods References
-
- Ashok B, Arleth L, Hjelm RP, Rubinstein I, Ny Ksel H. In vitro characterization of PEGylated phospholipid micelles for improved drug solubilization: Effects of PEG chain length and PC incorporation. Journal of Pharmaceutical Sciences. 2004;93:2476–2487. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

