CDK/CK1 inhibitors roscovitine and CR8 downregulate amplified MYCN in neuroblastoma cells
- PMID: 24317512
- PMCID: PMC4087096
- DOI: 10.1038/onc.2013.513
CDK/CK1 inhibitors roscovitine and CR8 downregulate amplified MYCN in neuroblastoma cells
Abstract
To understand the mechanisms of action of (R)-roscovitine and (S)-CR8, two related pharmacological inhibitors of cyclin-dependent kinases (CDKs), we applied a variety of '-omics' techniques to the human neuroblastoma SH-SY5Y and IMR32 cell lines: (1) kinase interaction assays, (2) affinity competition on immobilized broad-spectrum kinase inhibitors, (3) affinity chromatography on immobilized (R)-roscovitine and (S)-CR8, (4) whole genome transcriptomics analysis and specific quantitative PCR studies, (5) global quantitative proteomics approach and western blot analysis of selected proteins. Altogether, the results show that the major direct targets of these two molecules belong to the CDKs (1,2,5,7,9,12), DYRKs, CLKs and CK1s families. By inhibiting CDK7, CDK9 and CDK12, these inhibitors transiently reduce RNA polymerase 2 activity, which results in downregulation of a large set of genes. Global transcriptomics and proteomics analysis converge to a central role of MYC transcription factors downregulation. Indeed, CDK inhibitors trigger rapid and massive downregulation of MYCN expression in MYCN-amplified neuroblastoma cells as well as in nude mice xenografted IMR32 cells. Inhibition of casein kinase 1 may also contribute to the antitumoral activity of (R)-roscovitine and (S)-CR8. This dual mechanism of action may be crucial in the use of these kinase inhibitors for the treatment of MYC-dependent cancers, in particular neuroblastoma where MYCN amplification is a strong predictor factor for high-risk disease.
Figures
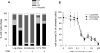
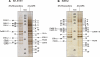
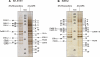









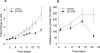
Similar articles
-
CR8, a potent and selective, roscovitine-derived inhibitor of cyclin-dependent kinases.Oncogene. 2008 Oct 2;27(44):5797-807. doi: 10.1038/onc.2008.191. Epub 2008 Jun 23. Oncogene. 2008. PMID: 18574471
-
The aurora kinase inhibitor CCT137690 downregulates MYCN and sensitizes MYCN-amplified neuroblastoma in vivo.Mol Cancer Ther. 2011 Nov;10(11):2115-23. doi: 10.1158/1535-7163.MCT-11-0333. Epub 2011 Sep 1. Mol Cancer Ther. 2011. PMID: 21885865 Free PMC article.
-
Inactivation of CDK2 is synthetically lethal to MYCN over-expressing cancer cells.Proc Natl Acad Sci U S A. 2009 Aug 4;106(31):12968-73. doi: 10.1073/pnas.0901418106. Epub 2009 Jun 12. Proc Natl Acad Sci U S A. 2009. PMID: 19525400 Free PMC article.
-
MDM2 as MYCN transcriptional target: implications for neuroblastoma pathogenesis.Cancer Lett. 2005 Oct 18;228(1-2):21-7. doi: 10.1016/j.canlet.2005.01.050. Cancer Lett. 2005. PMID: 15927364 Review.
-
The MYCN oncoprotein as a drug development target.Cancer Lett. 2003 Jul 18;197(1-2):125-30. doi: 10.1016/s0304-3835(03)00096-x. Cancer Lett. 2003. PMID: 12880971 Review.
Cited by
-
Human embryonic stem cell-derived neural crest model unveils CD55 as a cancer stem cell regulator for therapeutic targeting in MYCN-amplified neuroblastoma.Neuro Oncol. 2022 Jun 1;24(6):872-885. doi: 10.1093/neuonc/noab241. Neuro Oncol. 2022. PMID: 34655293 Free PMC article.
-
Genetic and pharmacological inhibition of Cdk1 provides neuroprotection towards ischemic neuronal death.Cell Death Discov. 2018 Mar 16;4:43. doi: 10.1038/s41420-018-0044-7. eCollection 2018. Cell Death Discov. 2018. PMID: 29581894 Free PMC article.
-
Diabetic Kinome Inhibitors-A New Opportunity for β-Cells Restoration.Int J Mol Sci. 2021 Aug 23;22(16):9083. doi: 10.3390/ijms22169083. Int J Mol Sci. 2021. PMID: 34445786 Free PMC article. Review.
-
Phosphoproteome Analysis Reveals Differential Mode of Action of Sorafenib in Wildtype and Mutated FLT3 Acute Myeloid Leukemia (AML) Cells.Mol Cell Proteomics. 2017 Jul;16(7):1365-1376. doi: 10.1074/mcp.M117.067462. Epub 2017 Apr 27. Mol Cell Proteomics. 2017. PMID: 28450419 Free PMC article.
-
MCL-1 inhibition in cancer treatment.Onco Targets Ther. 2018 Oct 23;11:7301-7314. doi: 10.2147/OTT.S146228. eCollection 2018. Onco Targets Ther. 2018. PMID: 30425521 Free PMC article. Review.
References
-
- Weinmann H, Metternich R. Drug discovery process for kinase inhibitors. ChemBioChem. 2005;6:455–459. - PubMed
-
- Eglen RM, Reisine T. The current status of drug discovery against the human kinome. Assay Drug Dev Technol. 2009;7:22–43. - PubMed
-
- Eglen R, Reisine T. Drug discovery and the human kinome: recent trends. Pharmacol Ther. 2011;130:144–156. - PubMed
-
- Via MC. Kinase-Targeted Therapeutics: Development Pipelines, Challenges, and Opportunities. Cambridge Healthtech Institute; 2011. p. 124.
-
- Petrelli A, Giordano S. From single- to multi-target drugs in cancer therapy: when aspecificity becomes an advantage. Curr Med Chem. 2008;15:422–432. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

