Structural basis of the alternating-access mechanism in a bile acid transporter
- PMID: 24317697
- PMCID: PMC4142352
- DOI: 10.1038/nature12811
Structural basis of the alternating-access mechanism in a bile acid transporter
Abstract
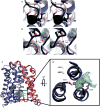
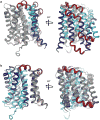
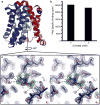
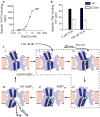
Bile acids are synthesized from cholesterol in hepatocytes and secreted through the biliary tract into the small intestine, where they aid in absorption of lipids and fat-soluble vitamins. Through a process known as enterohepatic recirculation, more than 90% of secreted bile acids are then retrieved from the intestine and returned to the liver for resecretion. In humans, there are two Na(+)-dependent bile acid transporters involved in enterohepatic recirculation, the Na(+)-taurocholate co-transporting polypeptide (NTCP; also known as SLC10A1) expressed in hepatocytes, and the apical sodium-dependent bile acid transporter (ASBT; also known as SLC10A2) expressed on enterocytes in the terminal ileum. In recent years, ASBT has attracted much interest as a potential drug target for treatment of hypercholesterolaemia, because inhibition of ASBT reduces reabsorption of bile acids, thus increasing bile acid synthesis and consequently cholesterol consumption. However, a lack of three-dimensional structures of bile acid transporters hampers our ability to understand the molecular mechanisms of substrate selectivity and transport, and to interpret the wealth of existing functional data. The crystal structure of an ASBT homologue from Neisseria meningitidis (ASBT(NM)) in detergent was reported recently, showing the protein in an inward-open conformation bound to two Na(+) and a taurocholic acid. However, the structural changes that bring bile acid and Na(+) across the membrane are difficult to infer from a single structure. To understand the structural changes associated with the coupled transport of Na(+) and bile acids, here we solved two structures of an ASBT homologue from Yersinia frederiksenii (ASBTYf) in a lipid environment, which reveal that a large rigid-body rotation of a substrate-binding domain gives the conserved 'crossover' region, where two discontinuous helices cross each other, alternating accessibility from either side of the cell membrane. This result has implications for the location and orientation of the bile acid during transport, as well as for the translocation pathway for Na(+).
Figures








References
-
- West KL, Ramjiganesh T, Roy S, Keller BT, Fernandez ML. 1-[4-[4[(4R,5R)-3,3-Dibutyl-7-(dimethylamino)-2,3,4,5-tetrahydro-4-hydroxy-1,1-di oxido-1-benzothiepin-5-yl]phenoxy]butyl]-4-aza-1-azoniabicyclo[2.2.2]octane methanesulfonate (SC-435), an ileal apical sodium-codependent bile acid transporter inhibitor alters hepatic cholesterol metabolism and lowers plasma low-density lipoprotein-cholesterol concentrations in guinea pigs. The Journal of pharmacology and experimental therapeutics. 2002;303:293–299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

