Herpes simplex virus encephalitis is a trigger of brain autoimmunity
- PMID: 24318406
- PMCID: PMC3961499
- DOI: 10.1002/ana.24083
Herpes simplex virus encephalitis is a trigger of brain autoimmunity
Abstract
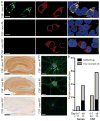
In 5 prospectively diagnosed patients with relapsing post-herpes simplex encephalitis (HSE), N-methyl-D-aspartate receptor (NMDAR) antibodies were identified. Antibody synthesis started 1 to 4 weeks after HSE, preceding the neurological relapse. Three of 5 patients improved postimmunotherapy, 1 spontaneously, and 1 has started to improve. Two additional patients with NMDAR antibodies, 9 with unknown neuronal surface antibodies, and 1 with NMDAR and unknown antibodies, were identified during retrospective assessment of 34 HSE patients; the frequency of autoantibodies increased over time (serum, p=0.004; cerebrospinal fluid, p=0.04). The 3 retrospectively identified NMDAR antibody-positive patients also had evidence of relapsing post-HSE. Overall, these findings indicate that HSE triggers NMDAR antibodies and potentially other brain autoimmunity.
© 2014 American Neurological Association.
Figures

References
-
- Sköldenberg B, Aurelius E, Hjalmarsson A, et al. Incidence and pathogenesis of clinical relapse after herpes simplex encephalitis in adults. J Neurol. 2006;253(2):163–170. - PubMed
-
- Schleede L, Bueter W, Baumgartner-Sigl S, et al. Pediatric Herpes Simplex Virus Encephalitis: A Retrospective Multicenter Experience. J Child Neurol. 2013;28(3):321–331. - PubMed
-
- De Tiège X, Rozenberg F, Portes Des V, et al. Herpes simplex encephalitis relapses in children: differentiation of two neurologic entities. Neurology. 2003;61(2):241–243. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

