Hepatic apolipoprotein M (apoM) overexpression stimulates formation of larger apoM/sphingosine 1-phosphate-enriched plasma high density lipoprotein
- PMID: 24318881
- PMCID: PMC3908412
- DOI: 10.1074/jbc.M113.499913
Hepatic apolipoprotein M (apoM) overexpression stimulates formation of larger apoM/sphingosine 1-phosphate-enriched plasma high density lipoprotein
Abstract
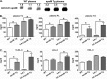
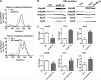
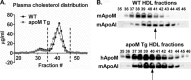
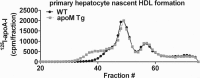
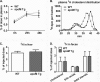
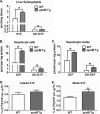
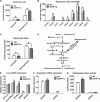
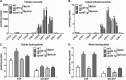
Apolipoprotein M (apoM), a lipocalin family member, preferentially associates with plasma HDL and binds plasma sphingosine 1-phosphate (S1P), a signaling molecule active in immune homeostasis and endothelial barrier function. ApoM overexpression in ABCA1-expressing HEK293 cells stimulated larger nascent HDL formation, compared with cells that did not express apoM; however, the in vivo role of apoM in HDL metabolism remains poorly understood. To test whether hepatic apoM overexpression increases plasma HDL size, we generated hepatocyte-specific apoM transgenic (APOM Tg) mice, which had an ∼3-5-fold increase in plasma apoM levels compared with wild-type mice. Although HDL cholesterol concentrations were similar to wild-type mice, APOM Tg mice had larger plasma HDLs enriched in apoM, cholesteryl ester, lecithin:cholesterol acyltransferase, and S1P. Despite the presence of larger plasma HDLs in APOM Tg mice, in vivo macrophage reverse cholesterol transport capacity was similar to that in wild-type mice. APOM Tg mice had an ∼5-fold increase in plasma S1P, which was predominantly associated with larger plasma HDLs. Primary hepatocytes from APOM Tg mice generated larger nascent HDLs and displayed increased sphingolipid synthesis and S1P secretion. Inhibition of ceramide synthases in hepatocytes increased cellular S1P levels but not S1P secretion, suggesting that apoM is rate-limiting in the export of hepatocyte S1P. Our data indicate that hepatocyte-specific apoM overexpression generates larger nascent HDLs and larger plasma HDLs, which preferentially bind apoM and S1P, and stimulates S1P biosynthesis for secretion. The unique apoM/S1P-enriched plasma HDL may serve to deliver S1P to extrahepatic tissues for atheroprotection and may have other as yet unidentified functions.
Keywords: ApoM; Apolipoproteins; Hepatocyte; High Density Lipoprotein (HDL); Liver; Sphingolipid; Sphingosine 1-Phosphate.
Figures


References
-
- AIM-HIGH Investigators, Boden W. E., Probstfield J. L., Anderson T., Chaitman B. R., Desvignes-Nickens P., Koprowicz K., McBride R., Teo K., Weintraub W. (2011) Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 365, 2255–2267 - PubMed
-
- Voight B. F., Peloso G. M., Orho-Melander M., Frikke-Schmidt R., Barbalic M., Jensen M. K., Hindy G., Hólm H., Ding E. L., Johnson T., Schunkert H., Samani N. J., Clarke R., Hopewell J. C., Thompson J. F., Li M., Thorleifsson G., Newton-Cheh C., Musunuru K., Pirruccello J. P., Saleheen D., Chen L., Stewart A., Schillert A., Thorsteinsdottir U., Thorgeirsson G., Anand S., Engert J. C., Morgan T., Spertus J., Stoll M., Berger K., Martinelli N., Girelli D., McKeown P. P., Patterson C. C., Epstein S. E., Devaney J., Burnett M. S., Mooser V., Ripatti S., Surakka I., Nieminen M. S., Sinisalo J., Lokki M. L., Perola M., Havulinna A., de Faire U., Gigante B., Ingelsson E., Zeller T., Wild P., de Bakker P. I., Klungel O. H., Maitland-van der Zee A. H., Peters B. J., de Boer A., Grobbee D. E., Kamphuisen P. W., Deneer V. H., Elbers C. C., Onland-Moret N. C., Hofker M. H., Wijmenga C., Verschuren W. M., Boer J. M., van der Schouw Y. T., Rasheed A., Frossard P., Demissie S., Willer C., Do R., Ordovas J. M., Abecasis G. R., Boehnke M., Mohlke K. L., Daly M. J., Guiducci C., Burtt N. P., Surti A., Gonzalez E., Purcell S., Gabriel S., Marrugat J., Peden J., Erdmann J., Diemert P., Willenborg C., König I. R., Fischer M., Hengstenberg C., Ziegler A., Buysschaert I., Lambrechts D., Van de Werf F., Fox K. A., El Mokhtari N. E., Rubin D., Schrezenmeir J., Schreiber S., Schäfer A., Danesh J., Blankenberg S., Roberts R., McPherson R., Watkins H., Hall A. S., Overvad K., Rimm E., Boerwinkle E., Tybjaerg-Hansen A., Cupples L. A., Reilly M. P., Melander O., Mannucci P. M., Ardissino D., Siscovick D., Elosua R., Stefansson K., O'Donnell C. J., Salomaa V., Rader D. J., Peltonen L., Schwartz S. M., Altshuler D., Kathiresan S. (2012) Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet 380, 572–580 - PMC - PubMed
-
- Glomset J. A. (1968) The plasma lecithins:cholesterol acyltransferase reaction. J. Lipid Res. 9, 155–167 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37GM043880/GM/NIGMS NIH HHS/United States
- P01HL49373/HL/NHLBI NIH HHS/United States
- R01 HL127649/HL/NHLBI NIH HHS/United States
- R01 GM043880/GM/NIGMS NIH HHS/United States
- R01HL119962/HL/NHLBI NIH HHS/United States
- P30 CA012197/CA/NCI NIH HHS/United States
- R01 HL064163/HL/NHLBI NIH HHS/United States
- 5P30CA12197/CA/NCI NIH HHS/United States
- R01 HL119962/HL/NHLBI NIH HHS/United States
- R01 HL112270/HL/NHLBI NIH HHS/United States
- R01 HL64163/HL/NHLBI NIH HHS/United States
- R01 CA061774/CA/NCI NIH HHS/United States
- R37 GM043880/GM/NIGMS NIH HHS/United States
- P30 CA016059/CA/NCI NIH HHS/United States
- P01 HL049373/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

