Interrogating congenital heart defects with noninvasive fetal echocardiography in a mouse forward genetic screen
- PMID: 24319090
- PMCID: PMC3962690
- DOI: 10.1161/CIRCIMAGING.113.000451
Interrogating congenital heart defects with noninvasive fetal echocardiography in a mouse forward genetic screen
Abstract
Background: Congenital heart disease (CHD) has a multifactorial pathogenesis, but a genetic contribution is indicated by heritability studies. To investigate the spectrum of CHD with a genetic pathogenesis, we conducted a forward genetic screen in inbred mice using fetal echocardiography to recover mutants with CHD. Mice are ideally suited for these studies given that they have the same four-chamber cardiac anatomy that is the substrate for CHD.
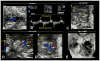
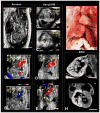
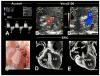
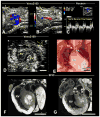
Methods and results: Ethylnitrosourea mutagenized mice were ultrasound-interrogated by fetal echocardiography using a clinical ultrasound system, and fetuses suspected to have cardiac abnormalities were further interrogated with an ultrahigh-frequency ultrasound biomicroscopy. Scanning of 46 270 fetuses revealed 1722 with cardiac anomalies, with 27.9% dying prenatally. Most of the structural heart defects can be diagnosed using ultrasound biomicroscopy but not with the clinical ultrasound system. Confirmation with analysis by necropsy and histopathology showed excellent diagnostic capability of ultrasound biomicroscopy for most CHDs. Ventricular septal defect was the most common CHD observed, whereas outflow tract and atrioventricular septal defects were the most prevalent complex CHD. Cardiac/visceral organ situs defects were observed at surprisingly high incidence. The rarest CHD found was hypoplastic left heart syndrome, a phenotype never seen in mice previously.
Conclusions: We developed a high-throughput, 2-tier ultrasound phenotyping strategy for efficient recovery of even rare CHD phenotypes, including the first mouse models of hypoplastic left heart syndrome. Our findings support a genetic pathogenesis for a wide spectrum of CHDs and suggest that the disruption of left-right patterning may play an important role in CHD.
Keywords: heart defects, congenital; microscopy, acoustic.
Figures




References
-
- Justice MJ. Capitalizing on large-scale mouse mutagenesis screens. Nat Rev Genet. 2000;1:109–115. - PubMed
-
- Shen Y, Leatherbury L, Rosenthal J, Yu Q, Pappas MA, Wessels A, Lucas J, Siegfried B, Chatterjee B, Svenson K, Lo CW. Cardiovascular phenotyping of fetal mice by noninvasive high-frequency ultrasound facilitates recovery of ENU-induced mutations causing congenital cardiac and extracardiac defects. Physiol Genomics. 2005;24:23–36. - PubMed
-
- Yu Q, Shen Y, Chatterjee B, Siegfried BH, Leatherbury L, Rosenthal J, Lucas JF, Wessels A, Spurney CF, Wu YJ, Kirby ML, Svenson K, Lo CW. ENU induced mutations causing congenital cardiovascular anomalies. Development. 2004;131:6211–6223. - PubMed
-
- Rosenthal J, Mangal V, Walker D, Bennett M, Mohun TJ, Lo CW. Rapid high resolution three dimensional reconstruction of embryos with episcopic fluorescence image capture. Birth Defects Res C Embryo Today. 2004;72:213–223. - PubMed
-
- Tobita K, Liu X, Lo CW. Imaging modalities to assess structural birth defects in mutant mouse models. Birth Defects Res C Embryo Today. 2010;90:176–184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

