A missense mutation in ITGB6 causes pitted hypomineralized amelogenesis imperfecta
- PMID: 24319098
- PMCID: PMC3959822
- DOI: 10.1093/hmg/ddt616
A missense mutation in ITGB6 causes pitted hypomineralized amelogenesis imperfecta
Abstract
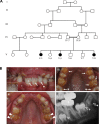
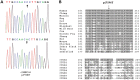
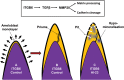
We identified a family in which pitted hypomineralized amelogenesis imperfecta (AI) with premature enamel failure segregated in an autosomal recessive fashion. Whole-exome sequencing revealed a missense mutation (c.586C>A, p.P196T) in the I-domain of integrin-β6 (ITGB6), which is consistently predicted to be pathogenic by all available programmes and is the only variant that segregates with the disease phenotype. Furthermore, a recent study revealed that mice lacking a functional allele of Itgb6 display a hypomaturation AI phenotype. Phenotypic characterization of affected human teeth in this study showed areas of abnormal prismatic organization, areas of low mineral density and severe abnormal surface pitting in the tooth's coronal portion. We suggest that the pathogenesis of this form of AI may be due to ineffective ligand binding of ITGB6 resulting in either compromised cell-matrix interaction or compromised ITGB6 activation of transforming growth factor-β (TGF-β) impacting indirectly on ameloblast-ameloblast interactions and proteolytic processing of extracellular matrix proteins via MMP20. This study adds to the list of genes mutated in AI and further highlights the importance of cell-matrix interactions during enamel formation.
Figures


References
-
- Bailleul-Forestier I., Molla M., Verloes A., Berdal A. The genetic basis of inherited anomalies of the teeth. Part 1: clinical and molecular aspects of non-syndromic dental disorders. Eur. J. Med. Genet. 2008;51:273–291. - PubMed
-
- Coffield K.D., Phillips C., Brady M., Roberts M.W., Strauss R.P., Wright J.T. The psychosocial impact of developmental dental defects in people with hereditary amelogenesis imperfecta. J. Am. Dent. Assoc. 2005;136:620–630. - PubMed
-
- Robinson C., Kirkham J., Weatherell J.A., Richards A., Josephsen K., Fejerskov O. Mineral and protein concentrations in enamel of the developing permanent porcine dentition. Caries Res. 1988;22:321–326. - PubMed
-
- Smith C.E. Cellular and chemical events during enamel maturation. Crit. Rev. Oral Biol. Med. 1998;9:128–161. - PubMed
-
- Sawada T., Yamamoto T., Yanagisawa T., Takuma S., Hasegawa H., Watanabe K. Evidence for uptake of basement membrane by differentiating ameloblasts in the rat incisor enamel organ. J. Dent. Res. 1990;69:1508–1511. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

