Sidedness of carbamazepine accessibility to voltage-gated sodium channels
- PMID: 24319110
- PMCID: PMC3913360
- DOI: 10.1124/mol.113.090472
Sidedness of carbamazepine accessibility to voltage-gated sodium channels
Abstract
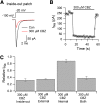
Voltage-gated sodium channels are inhibited by many local anesthetics, antiarrhythmics, and antiepileptic drugs. The local anesthetic lidocaine appears to be able to access its binding site in the sodium channel only from the membrane phase or from the internal face of the channel. In contrast, the antiepileptic drug carbamazepine was found to inhibit voltage-gated sodium channels only with external, but not internal, application, implying a major difference. We investigated this point using both whole-cell and inside-out patch recordings from human Na(v)1.7 channels in a stable cell line. In the whole-cell configuration, carbamazepine inhibited sodium current within seconds when applied externally, but had little or no effect when applied internally for up to 15 minutes, confirming previous results. However, carbamazepine inhibited sodium channels effectively and rapidly when applied to the internal face of the membrane using inside-out patch recording. We found that lidocaine also has little or no effect when applied intracellularly in whole-cell recording, but blocks effectively and rapidly when applied to the internal surface using inside-out patches. In contrast, the cationic lidocaine derivative QX-314 (N-ethyl-lidocaine) blocks effectively when applied internally with whole-cell dialysis, as well as when applied to inside-out patches. We conclude that carbamazepine and lidocaine access the sodium channel in similar ways and hypothesize that their lack of effect with internal dialysis in whole-cell recording reflects rapid exit through membrane near the pipette recording site. This effect likely limits the ability of any compound with significant membrane permeability to be applied intracellularly by whole-cell dialysis.
Figures



References
-
- Frazier DT, Narahashi T, Yamada M. (1970) The site of action and active form of local anesthetics. II. Experiments with quaternary compounds. J Pharmacol Exp Ther 171:45–51 - PubMed
-
- Khodorov B, Shishkova L, Peganov E, Revenko S. (1976) Inhibition of sodium currents in frog Ranvier node treated with local anesthetics. Role of slow inactivation. Biochim Biophys Acta 433:409–435
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

