Regulation of β2-adrenergic receptor function by conformationally selective single-domain intrabodies
- PMID: 24319111
- PMCID: PMC3935154
- DOI: 10.1124/mol.113.089516
Regulation of β2-adrenergic receptor function by conformationally selective single-domain intrabodies
Abstract
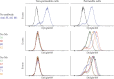
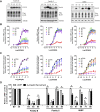
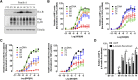
The biologic activity induced by ligand binding to orthosteric or allosteric sites on a G protein-coupled receptor (GPCR) is mediated by stabilization of specific receptor conformations. In the case of the β2 adrenergic receptor, these ligands are generally small-molecule agonists or antagonists. However, a monomeric single-domain antibody (nanobody) from the Camelid family was recently found to allosterically bind and stabilize an active conformation of the β2-adrenergic receptor (β2AR). Here, we set out to study the functional interaction of 18 related nanobodies with the β2AR to investigate their roles as novel tools for studying GPCR biology. Our studies revealed several sequence-related nanobody families with preferences for active (agonist-occupied) or inactive (antagonist-occupied) receptors. Flow cytometry analysis indicates that all nanobodies bind to epitopes displayed on the intracellular receptor surface; therefore, we transiently expressed them intracellularly as "intrabodies" to test their effects on β2AR-dependent signaling. Conformational specificity was preserved after intrabody conversion as demonstrated by the ability for the intracellularly expressed nanobodies to selectively bind agonist- or antagonist-occupied receptors. When expressed as intrabodies, they inhibited G protein activation (cyclic AMP accumulation), G protein-coupled receptor kinase (GRK)-mediated receptor phosphorylation, β-arrestin recruitment, and receptor internalization to varying extents. These functional effects were likely due to either steric blockade of downstream effector (Gs, β-arrestin, GRK) interactions or stabilization of specific receptor conformations which do not support effector coupling. Together, these findings strongly implicate nanobody-derived intrabodies as novel tools to study GPCR biology.
Figures


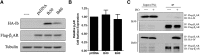
References
-
- Barlow JN, Conrath K, Steyaert J. (2009) Substrate-dependent modulation of enzyme activity by allosteric effector antibodies. Biochim Biophys Acta 1794:1259–1268 - PubMed
-
- Benovic JL, Kühn H, Weyand I, Codina J, Caron MG, Lefkowitz RJ. (1987) Functional desensitization of the isolated beta-adrenergic receptor by the beta-adrenergic receptor kinase: potential role of an analog of the retinal protein arrestin (48-kDa protein). Proc Natl Acad Sci USA 84:8879–8882 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

