A cypovirus VP5 displays the RNA chaperone-like activity that destabilizes RNA helices and accelerates strand annealing
- PMID: 24319147
- PMCID: PMC3936753
- DOI: 10.1093/nar/gkt1256
A cypovirus VP5 displays the RNA chaperone-like activity that destabilizes RNA helices and accelerates strand annealing
Abstract
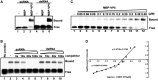
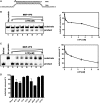
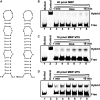
For double-stranded RNA (dsRNA) viruses in the family Reoviridae, their inner capsids function as the machinery for viral RNA (vRNA) replication. Unlike other multishelled reoviruses, cypovirus has a single-layered capsid, thereby representing a simplified model for studying vRNA replication of reoviruses. VP5 is one of the three major cypovirus capsid proteins and functions as a clamp protein to stabilize cypovirus capsid. Here, we expressed VP5 from type 5 Helicoverpa armigera cypovirus (HaCPV-5) in a eukaryotic system and determined that this VP5 possesses RNA chaperone-like activity, which destabilizes RNA helices and accelerates strand annealing independent of ATP. Our further characterization of VP5 revealed that its helix-destabilizing activity is RNA specific, lacks directionality and could be inhibited by divalent ions, such as Mg(2+), Mn(2+), Ca(2+) or Zn(2+), to varying degrees. Furthermore, we found that HaCPV-5 VP5 facilitates the replication initiation of an alternative polymerase (i.e. reverse transcriptase) through a panhandle-structured RNA template, which mimics the 5'-3' cyclization of cypoviral positive-stranded RNA. Given that the replication of negative-stranded vRNA on the positive-stranded vRNA template necessitates the dissociation of the 5'-3' panhandle, the RNA chaperone activity of VP5 may play a direct role in the initiation of reoviral dsRNA synthesis.
Figures









References
-
- Mertens P. The dsRNA viruses. Virus Res. 2004;101:3–13. - PubMed
-
- Reinisch KM. The dsRNA Viridae and their catalytic capsids. Nat. Struct. Biol. 2002;9:714–716. - PubMed
-
- Xia Q, Jakana J, Zhang JQ, Zhou ZH. Structural comparisons of empty and full cytoplasmic polyhedrosis virus. Protein-RNA interactions and implications for endogenous RNA transcription mechanism. J. Biol. Chem. 2003;278:1094–1100. - PubMed
-
- Poranen MM, Bamford DH. In: Viral Molecular Machines. Rossmann MG, Rao VB, editors. Springer, New York, NY, USA: Science; 2012. pp. 379–402.
-
- King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ. Virus taxonomy: ninth report of the International Committee on Taxonomy of Viruses. Vol. 9. Academic Press, Salt Lake City, UT, USA: Elsevier; 2011. pp. 497–650.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

