A CD38/CD203a/CD73 ectoenzymatic pathway independent of CD39 drives a novel adenosinergic loop in human T lymphocytes
- PMID: 24319640
- PMCID: PMC3850273
- DOI: 10.4161/onci.26246
A CD38/CD203a/CD73 ectoenzymatic pathway independent of CD39 drives a novel adenosinergic loop in human T lymphocytes
Abstract
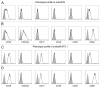
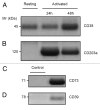
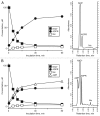
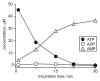
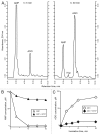
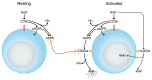
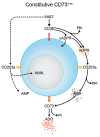
The tumor microenvironment is characterized by of high levels of extracellular nucleotides that are metabolized through the dynamic and sequential action of cell surface enzymes (ectoenzymes). These ectoenzymes operate according to their spatial arrangement, as part of (1) continuous (molecules on the same cell) or (2) discontinuous (molecules on different cells) pathways, the latter being facilitated by restricted cellular microenvironment. The outcome of this catabolic activity is an increase in the local concentration of adenosine, a nucleoside involved in the control of inflammation and immune responses. The aim of the work presented here was to demonstrate that a previously unexplored enzymatic pathway may be an alternate route to produce extracellular adenosine. Our data show that this new axis is driven by the nucleotide-metabolizing ectoenzymes CD38 (an NAD+ nucleosidase), the ecto-nucleotide pyrophosphatase/phosphodiesterase 1 (NPP1, also known as CD203a or PC-1) and the 5' ectonucleotidase (5'-NT) CD73, while bypassing the canonical catabolic pathway mediated by the nucleoside tri- and diphosphohydrolase (NTPDase) CD39. To determine the relative contributions of these cell surface enzymes to the production of adenosine, we exploited a human T-cell model allowing for the modular expression of the individual components of this alternative pathway upon activation and transfection. The biochemical analysis of the products of these ectoenzymes by high-performance liquid chromatography (HPLC) fully substantiated our working hypothesis. This newly characterized pathway may facilitate the emergence of an adaptive immune response in selected cellular contexts. Considering the role for extracellular adenosine in the regulation of inflammation and immunogenicity, this pathway could constitute a novel strategy of tumor evasion, implying that these enzymes may represent ideal targets for antibody-mediated therapy.
Keywords: CD203a; CD38; NAD+; PC-1; adenosine; ectonucleotidases.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
