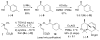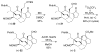Total synthesis of (-)-calyciphylline N
- PMID: 24319987
- PMCID: PMC3908864
- DOI: 10.1021/ja411539w
Total synthesis of (-)-calyciphylline N
Abstract
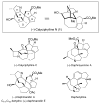
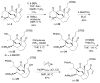
The total synthesis of the architecturally complex Daphniphyllum alkaloid (-)-calyciphylline N has been achieved. Highlights of the synthesis include a Et2AlCl-promoted, highly stereoselective, susbtrate-controlled intramolecular Diels-Alder reaction, a transannular enolate alkylation, an effective Stille carbonylation/Nazarov cyclization sequence, and a high-risk diastereoselective hydrogenation of a fully substituted conjugated diene ester.
Figures
References
-
- Kobayashi J, Kubota T. Nat Prod Rep. 2009;26:936. - PubMed
-
-
For recent synthetic studies, see: Coldham I, Watson L, Adams H, Martin NG. J Org Chem. 2011;76:2360.Darses B, Michaelides IN, Sladojevich F, Ward JW, Rzepa PR, Dixon DJ. Org Lett. 2012;14:1684.
-
-
-
For an elegant total synthesis of the closely related congener, (+)-daphmanidin E (Figure 1), see: Weiss ME, Carreira EM. Angew Chem Int Ed. 2011;50:11501.
-
-
-
For a very recent total synthesis of the Daphniphyllum alkaloid, daphenylline (Figure 1), see: Lu ZY, Li Y, Deng J, Li A. Nat Chem. 2013;5:679.
-
-
- Yahata H, Kubota T, Kobayashi J. J Nat Prod. 2008;72:148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources