Mitochondrial function and energy metabolism in neuronal HT22 cells resistant to oxidative stress
- PMID: 24319993
- PMCID: PMC3976627
- DOI: 10.1111/bph.12549
Mitochondrial function and energy metabolism in neuronal HT22 cells resistant to oxidative stress
Abstract
Background and purpose: The hippocampal cell line HT22 is an excellent model for studying the consequences of endogenous oxidative stress. Extracellular glutamate depletes cellular glutathione by blocking the glutamate/cystine antiporter system xc-. Glutathione depletion induces a well-defined programme of cell death characterized by an increase in reactive oxygen species and mitochondrial dysfunction.
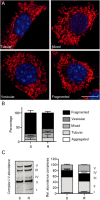
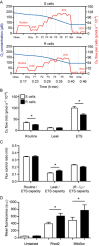
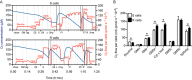
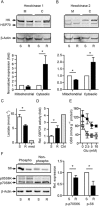
Experimental approach: We compared the mitochondrial shape, the abundance of mitochondrial complexes and the mitochondrial respiration of HT22 cells, selected based on their resistance to glutamate, with those of the glutamate-sensitive parental cell line.
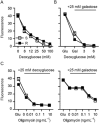
Key results: Glutamate-resistant mitochondria were less fragmented and displayed seemingly contradictory features: mitochondrial calcium and superoxide were increased while high-resolution respirometry suggested a reduction in mitochondrial respiration. This was interpreted as a reverse activity of the ATP synthase under oxidative stress, leading to hydrolysis of ATP to maintain or even elevate the mitochondrial membrane potential, suggesting these cells endure ineffective energy metabolism to protect their membrane potential. Glutamate-resistant cells were also resistant to oligomycin, an inhibitor of the ATP synthase, but sensitive to deoxyglucose, an inhibitor of hexokinases. Exchanging glucose with galactose rendered resistant cells 1000-fold more sensitive to oligomycin. These results, together with a strong increase in cytosolic hexokinase 1 and 2, a reduced lactate production and an increased activity of glucose-6-phosphate dehydrogenase, suggest that glutamate-resistant HT22 cells shuttle most available glucose towards the hexose monophosphate shunt to increase glutathione recovery.
Conclusions and implications: These results indicate that mitochondrial and metabolic adaptations play an important role in the resistance of cells to oxidative stress.
Keywords: cell death; fusion/fission; glycolysis; mitochondria; oxidative phosphorylation; oxidative stress.
© 2013 The British Pharmacological Society.
Figures

References
-
- Albrecht P, Lewerenz J, Dittmer S, Noack R, Maher P, Methner A. Mechanisms of oxidative glutamate toxicity: the glutamate/cystine antiporter system xc− as a neuroprotective drug target. CNS Neurol Disord Drug Targets. 2010;9:373–382. - PubMed
-
- Ben-Yoseph O, Boxer PA, Ross BD. Assessment of the role of the glutathione and pentose phosphate pathways in the protection of primary cerebrocortical cultures from oxidative stress. J Neurochem. 1996;66:2329–2337. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

