DNA transposon-based gene vehicles - scenes from an evolutionary drive
- PMID: 24320156
- PMCID: PMC3878927
- DOI: 10.1186/1423-0127-20-92
DNA transposon-based gene vehicles - scenes from an evolutionary drive
Abstract
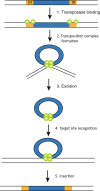
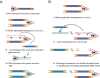
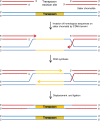
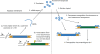
DNA transposons are primitive genetic elements which have colonized living organisms from plants to bacteria and mammals. Through evolution such parasitic elements have shaped their host genomes by replicating and relocating between chromosomal loci in processes catalyzed by the transposase proteins encoded by the elements themselves. DNA transposable elements are constantly adapting to life in the genome, and self-suppressive regulation as well as defensive host mechanisms may assist in buffering 'cut-and-paste' DNA mobilization until accumulating mutations will eventually restrict events of transposition. With the reconstructed Sleeping Beauty DNA transposon as a powerful engine, a growing list of transposable elements with activity in human cells have moved into biomedical experimentation and preclinical therapy as versatile vehicles for delivery and genomic insertion of transgenes. In this review, we aim to link the mechanisms that drive transposon evolution with the realities and potential challenges we are facing when adapting DNA transposons for gene transfer. We argue that DNA transposon-derived vectors may carry inherent, and potentially limiting, traits of their mother elements. By understanding in detail the evolutionary journey of transposons, from host colonization to element multiplication and inactivation, we may better exploit the potential of distinct transposable elements. Hence, parallel efforts to investigate and develop distinct, but potent, transposon-based vector systems will benefit the broad applications of gene transfer. Insight and clever optimization have shaped new DNA transposon vectors, which recently debuted in the first DNA transposon-based clinical trial. Learning from an evolutionary drive may help us create gene vehicles that are safer, more efficient, and less prone for suppression and inactivation.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

