Integrated ultrasound and magnetic resonance imaging for simultaneous temperature and cavitation monitoring during focused ultrasound therapies
- PMID: 24320468
- PMCID: PMC3808488
- DOI: 10.1118/1.4823793
Integrated ultrasound and magnetic resonance imaging for simultaneous temperature and cavitation monitoring during focused ultrasound therapies
Abstract
Purpose: Ultrasound can be used to noninvasively produce different bioeffects via viscous heating, acoustic cavitation, or their combination, and these effects can be exploited to develop a wide range of therapies for cancer and other disorders. In order to accurately localize and control these different effects, imaging methods are desired that can map both temperature changes and cavitation activity. To address these needs, the authors integrated an ultrasound imaging array into an MRI-guided focused ultrasound (MRgFUS) system to simultaneously visualize thermal and mechanical effects via passive acoustic mapping (PAM) and MR temperature imaging (MRTI), respectively.
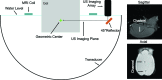
Methods: The system was tested with an MRgFUS system developed for transcranial sonication for brain tumor ablation in experiments with a tissue mimicking phantom and a phantom-filled ex vivo macaque skull. In experiments on cavitation-enhanced heating, 10 s continuous wave sonications were applied at increasing power levels (30-110 W) until broadband acoustic emissions (a signature for inertial cavitation) were evident. The presence or lack of signal in the PAM, as well as its magnitude and location, were compared to the focal heating in the MRTI. Additional experiments compared PAM with standard B-mode ultrasound imaging and tested the feasibility of the system to map cavitation activity produced during low-power (5 W) burst sonications in a channel filled with a microbubble ultrasound contrast agent.
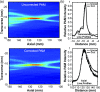
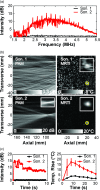
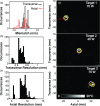
Results: When inertial cavitation was evident, localized activity was present in PAM and a marked increase in heating was observed in MRTI. The location of the cavitation activity and heating agreed on average after registration of the two imaging modalities; the distance between the maximum cavitation activity and focal heating was -3.4 ± 2.1 mm and -0.1 ± 3.3 mm in the axial and transverse ultrasound array directions, respectively. Distortions and other MRI issues introduced small uncertainties in the PAM∕MRTI registration. Although there was substantial variation, a nonlinear relationship between the average intensity of the cavitation maps, which was relatively constant during sonication, and the peak temperature rise was evident. A fit to the data to an exponential had a correlation coefficient (R(2)) of 0.62. The system was also found to be capable of visualizing cavitation activity with B-mode imaging and of passively mapping cavitation activity transcranially during cavitation-enhanced heating and during low-power sonication with an ultrasound contrast agent.
Conclusions: The authors have demonstrated the feasibility of integrating an ultrasound imaging array into an MRgFUS system to simultaneously map localized cavitation activity and temperature. The authors anticipate that this integrated approach can be utilized to develop controllers for cavitation-enhanced ablation and facilitate the optimization and development of this and other ultrasound therapies. The integrated system may also provide a useful tool to study the bioeffects of acoustic cavitation.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

