Mitochondrial uncoupling protein 2 induces cell cycle arrest and necrotic cell death
- PMID: 24320727
- PMCID: PMC3996969
- DOI: 10.1089/met.2013.0096
Mitochondrial uncoupling protein 2 induces cell cycle arrest and necrotic cell death
Abstract
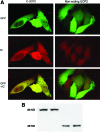
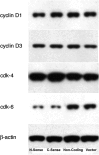
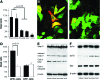
Uncoupling protein 2 (UCP2) is a mitochondrial membrane protein that regulates energy metabolism and reactive oxygen species (ROS) production. We generated mouse carboxy- and amino-terminal green fluorescent protein (GFP)-tagged UCP2 constructs to investigate the effect of UCP2 expression on cell proliferation and viability. UCP2-transfected Hepa 1-6 cells did not show reduced cellular adenosine triphosphate (ATP) but showed increased levels of glutathione. Flow cytometry analysis indicated that transfected cells were less proliferative than nontransfected controls, with most cells blocked at the G1 phase. The effect of UCP2 on cell cycle arrest could not be reversed by providing exogenous ATP or oxidant supply, and was not affected by the chemical uncoupler carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP). However, this effect of UCP2 was augmented by treatment with genistein, a tyrosine kinase inhibitor, which by itself did not affect cell proliferation on control hepatocytes. Western blotting analysis revealed decreased expression levels of CDK6 but not CDK2 and D-type cyclins. Examination of cell viability in UCP2-transfected cells with Trypan Blue and Annexin-V staining revealed that UCP2 transfection led to significantly increased cell death. However, characteristics of apoptosis were absent in UCP2-transfected Hepa 1-6 cells, including lack of oligonucleosomal fragmentation (laddering) of chromosomal DNA, release of cytochrome c from mitochondria, and cleavage of caspase-3. In conclusion, our results indicate that UCP2 induces cell cycle arrest at G1 phase and causes nonapoptotic cell death, suggesting that UCP2 may act as a powerful influence on hepatic regeneration and cell death in the steatotic liver.
Figures



References
-
- Stuart JA, Cadenas S, Jekabsons MB, et al. . Mitochondrial proton leak and the uncoupling protein 1 homologues. Biochim Biophys Acta 2001;1504:144–158 - PubMed
-
- Nicholls DG, Bernson VS, Heaton GM. The identification of the component in the inner membrane of brown adipose tissue mitochondria responsible for regulating energy dissipation. Experientia Suppl 1978;32:89–93 - PubMed
-
- Nicholls DG, Locke RM. Thermogenic mechanisms in brown fat. Physiol Rev 1984;64:1–64 - PubMed
-
- Boss O, Samec S, Paoloni-Giacobino A, et al. . Uncoupling protein-3: A new member of the mitochondrial carrier family with tissue-specific expression. FEBS Lett 1997;408:39–42 - PubMed
-
- Mao W, Yu XX, Zhong A, et al. . UCP4, a novel brain-specific mitochondrial protein that reduces membrane potential in mammalian cells. FEBS Lett 1999;443:326–330 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
