A multicentre clinical study on the injection of ceftriaxone/sulbactam compared with cefoperazone/sulbactam in the treatment of respiratory and urinary tract infections
- PMID: 24321187
- PMCID: PMC4029302
- DOI: 10.1186/1476-0711-12-38
A multicentre clinical study on the injection of ceftriaxone/sulbactam compared with cefoperazone/sulbactam in the treatment of respiratory and urinary tract infections
Abstract
Objective: This clinical study was designed to evaluate the efficacy and safety of this therapy in the treatment of respiratory and urinary infections caused by ceftriaxone-resistant bacteria in comparison with the effect of cefoperazone/sulbactam on cefoperazone-resistant bacteria.
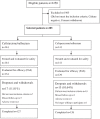
Methods: A total of 285 patients aged from 18 to 65 years old, with a respiratory or urinary tract bacterial infection, were enrolled into this multicentre, open-label, controlled clinical study, and bacteria that were either ceftriaxone-resistant or cefoperazone-resistant were isolated from the patients, whose condition had not improved after three days of treatment with ceftriaxone or cefoperazone. To be selected for the study, bacterial cultures obtained from the patients had to be positive before enrolment, and all of the isolates were required to be β-lactamase-positive. Of these patients, 253 completed the trial, and 263 were enrolled into the intention-to-treat (ITT) analysis. All of the 285 patients were included in the safety analysis.
Results: The cure and effective rates were 39.55% and 85.07% in the ceftriaxone/sulbactam group and 36.43% and 79.84% in the cefoperazone/sulbactam group; the bacterial eradication rates were 83.58% and 83.72%; and the adverse-event rates were 7.48% and 7.80%, respectively. There were no significant differences between the two groups (p > 0.05).
Conclusion: Ceftriaxone/sulbactam is as effective and well-tolerated as cefoperazone/sulbactam for the treatment of intermediate and severe bacterial infections caused by resistant strains.
References
-
- Xiao YH, Wang J, Yan Z, Qi HM, Xy L, Zhao CY. Mohnarin of 2008 et al.Surveillance results of national bacterial drug resistanc. Chin J Clin Pharmacol Therap. 2010;12(16):2377–2383.
-
- Akova M. Sulbactam-containing beta-lactamase inhibitor combinations. Clin Microbiol Infect. 2008;12(Suppl 1):185–188. - PubMed
-
- Shrivastava SM, Kumar S, Chaudhary M. Ceftriaxone-sulbactam combination: microbial analysis by variation of ratios and comparative disc diffusion. Curr Res Bacteriol. 2009;12:50–55. doi: 10.3923/crb.2009.50.55. - DOI
-
- Yonghong X, Yuan L, Zisheng K, Jan L. Tolerability and pharmacokinetics of multiple dose ceftriaxone/sulbactam injection in chinese. Chin J Clin Pharmacol Therap. 2006;12:458–506.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical