Synthesis, DNA binding and topoisomerase I inhibition activity of thiazacridine and imidazacridine derivatives
- PMID: 24322489
- PMCID: PMC6270168
- DOI: 10.3390/molecules181215035
Synthesis, DNA binding and topoisomerase I inhibition activity of thiazacridine and imidazacridine derivatives
Abstract
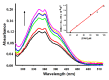
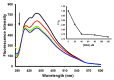
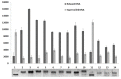
Thiazacridine and imidazacridine derivatives have shown promising results as tumors suppressors in some cancer cell lines. For a better understanding of the mechanism of action of these compounds, binding studies of 5-acridin-9-ylmethylidene-3-amino-2-thioxo-thiazolidin-4-one, 5-acridin-9-ylmethylidene-2-thioxo-thiazolidin-4-one, 5-acridin-9-ylmethylidene-2-thioxo-imidazolidin-4-one and 3-acridin-9-ylmethyl-thiazolidin-2,4-dione with calf thymus DNA (ctDNA) by electronic absorption and fluorescence spectroscopy and circular dichroism spectroscopy were performed. The binding constants ranged from 1.46 × 10(4) to 6.01 × 10(4) M(-1). UV-Vis, fluorescence and circular dichroism measurements indicated that the compounds interact effectively with ctDNA, both by intercalation or external binding. They demonstrated inhibitory activities to human topoisomerase I, except for 5-acridin-9-ylmethylidene-2-thioxo-1,3-thiazolidin-4-one. These results provide insight into the DNA binding mechanism of imidazacridines and thiazacridines.
Figures




References
-
- Mukherjee A., Lavery R., Bagchi B., Hynes J.T. On the molecular mechanism of drug intercalation into DNA: A simulation study of the intercalation pathway, free energy, and DNA structural changes. J. Am. Chem. Soc. 2008;130:9747–9755. - PubMed
-
- Hurley H.L. DNA and its associated processes as targets for cancer therapy. Nat. Rev. Cancer. 2002;2:188–200. - PubMed
-
- Ihmels H., Otto D. Intercalation of organic dye molecules into double-stranded DNA general principles and recent developments. Top. Curr. Chem. 2005;258:161–204.
-
- Barros F.W.A., Silva T.G., Pitta M.G.R., Bezerra D.P., Costa-Lotufo L.V., Moraes M.O., Pessoa C., Moura M.A.F.B., Abreu F.C., Lima M.C.A., et al. Synthesis and cytotoxic activity of new acridine-thiazolidine derivatives. Bioorg. Med. Chem. 2012;20:3533–3539. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

