The role of replicates for error mitigation in next-generation sequencing
- PMID: 24322726
- PMCID: PMC4103745
- DOI: 10.1038/nrg3655
The role of replicates for error mitigation in next-generation sequencing
Abstract
Advances in next-generation sequencing (NGS) technologies have rapidly improved sequencing fidelity and substantially decreased sequencing error rates. However, given that there are billions of nucleotides in a human genome, even low experimental error rates yield many errors in variant calls. Erroneous variants can mimic true somatic and rare variants, thus requiring costly confirmatory experiments to minimize the number of false positives. Here, we discuss sources of experimental errors in NGS and how replicates can be used to abate such errors.
Figures

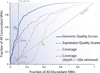
References
-
-
Metzker ML. Sequencing technologies - the next generation. Nat Rev Genet. 2010;11:31–46. This review details many current sequencing technologies, including their strengths and limitations.
-
-
-
Ratan A, et al. Comparison of sequencing platforms for single nucleotide variant calls in a human sample. PLoS One. 2013;8:e55089. A thorough study of current error modes, coverage profiles and GC-biases of Next-generation technologies.
-
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

