Tumor mismatch repair immunohistochemistry and DNA MLH1 methylation testing of patients with endometrial cancer diagnosed at age younger than 60 years optimizes triage for population-level germline mismatch repair gene mutation testing
- PMID: 24323032
- PMCID: PMC4876359
- DOI: 10.1200/JCO.2013.51.2129
Tumor mismatch repair immunohistochemistry and DNA MLH1 methylation testing of patients with endometrial cancer diagnosed at age younger than 60 years optimizes triage for population-level germline mismatch repair gene mutation testing
Abstract
Purpose: Clinicopathologic data from a population-based endometrial cancer cohort, unselected for age or family history, were analyzed to determine the optimal scheme for identification of patients with germline mismatch repair (MMR) gene mutations.
Patients and methods: Endometrial cancers from 702 patients recruited into the Australian National Endometrial Cancer Study (ANECS) were tested for MMR protein expression using immunohistochemistry (IHC) and for MLH1 gene promoter methylation in MLH1-deficient cases. MMR mutation testing was performed on germline DNA of patients with MMR-protein deficient tumors. Prediction of germline mutation status was compared for combinations of tumor characteristics, age at diagnosis, and various clinical criteria (Amsterdam, Bethesda, Society of Gynecologic Oncology, ANECS).
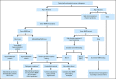
Results: Tumor MMR-protein deficiency was detected in 170 (24%) of 702 cases. Germline testing of 158 MMR-deficient cases identified 22 truncating mutations (3% of all cases) and four unclassified variants. Tumor MLH1 methylation was detected in 99 (89%) of 111 cases demonstrating MLH1/PMS2 IHC loss; all were germline MLH1 mutation negative. A combination of MMR IHC plus MLH1 methylation testing in women younger than 60 years of age at diagnosis provided the highest positive predictive value for the identification of mutation carriers at 46% versus ≤ 41% for any other criteria considered.
Conclusion: Population-level identification of patients with MMR mutation-positive endometrial cancer is optimized by stepwise testing for tumor MMR IHC loss in patients younger than 60 years, tumor MLH1 methylation in individuals with MLH1 IHC loss, and germline mutations in patients exhibiting loss of MSH6, MSH2, or PMS2 or loss of MLH1/PMS2 with absence of MLH1 methylation.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
Reply to J. Moline et al.J Clin Oncol. 2014 Jul 20;32(21):2278-9. doi: 10.1200/JCO.2014.55.8213. Epub 2014 Jun 9. J Clin Oncol. 2014. PMID: 24912891 No abstract available.
-
Equality in lynch syndrome screening: why should we hold patients with endometrial cancer to a different standard?J Clin Oncol. 2014 Jul 20;32(21):2277. doi: 10.1200/JCO.2014.55.3602. Epub 2014 Jun 9. J Clin Oncol. 2014. PMID: 24912902 No abstract available.
References
-
- Bonadona V, Bonaïti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305:2304–2310. - PubMed
-
- Engel C, Loeffler M, Steinke V, et al. Risks of less common cancers in proven mutation carriers with Lynch syndrome. J Clin Oncol. 2012;30:4409–4415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

