Plasmonic nanomaterials for biodiagnostics
- PMID: 24323079
- PMCID: PMC5414846
- DOI: 10.1039/c3cs60346f
Plasmonic nanomaterials for biodiagnostics
Abstract
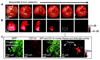
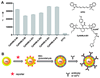
The application of nanomaterials to detect disease biomarkers is giving rise to ultrasensitive assays, with scientists exploiting the many advantageous physical and chemical properties of nanomaterials. The fundamental basis of such work is to link unique phenomena that arise at the nanoscale to the presence of a specific analyte biomolecule, and to modulate the intensity of such phenomena in a ratiometric fashion, in direct proportion with analyte concentration. Precise engineering of nanomaterial surfaces is of utmost importance here, as the interface between the material and the biological environment is where the key interactions occur. In this tutorial review, we discuss the use of plasmonic nanomaterials in the development of biodiagnostic tools for the detection of a large variety of biomolecular analytes, and how their plasmonic properties give rise to tunable optical characteristics and surface enhanced Raman signals. We put particular focus on studies that have explored the efficacy of the systems using physiological samples in an effort to highlight the clinical potential of such assays.
Figures











References
-
- Pelaz B, Jaber S, de Aberasturi DJ, Wulf V, Aida T, de la Fuente JM, Feldmann J, Gaub HE, Josephson L, Kagan CR, Kotov NA, et al. ACS Nano. 2012;6:8468–8483. - PubMed
-
- Wang J, Qu X. Nanoscale. 2013;5:3589–3600. - PubMed
-
- Kumar PS, Pastoriza-Santos I, Rodriguez-Gonzalez B, Garcia de Abajo FJ, Liz-Marzan LM. Nanotechnology. 2008;19:015606. - PubMed
-
- Gupta S, Andresen H, Ghadiali JE, Stevens MM. Small. 2010;6:1509–1513. - PubMed
-
- Aili D, Mager M, Roche D, Stevens MM. Nano Lett. 2011;11:1401–1405. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

