Chemotherapy activates cancer-associated fibroblasts to maintain colorectal cancer-initiating cells by IL-17A
- PMID: 24323355
- PMCID: PMC3865474
- DOI: 10.1084/jem.20131195
Chemotherapy activates cancer-associated fibroblasts to maintain colorectal cancer-initiating cells by IL-17A
Abstract
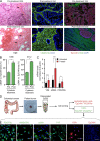
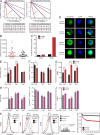
Many solid cancers display cellular hierarchies with self-renewing, tumorigenic stemlike cells, or cancer-initiating cells (CICs) at the apex. Whereas CICs often exhibit relative resistance to conventional cancer therapies, they also receive critical maintenance cues from supportive stromal elements that also respond to cytotoxic therapies. To interrogate the interplay between chemotherapy and CICs, we investigated cellular heterogeneity in human colorectal cancers. Colorectal CICs were resistant to conventional chemotherapy in cell-autonomous assays, but CIC chemoresistance was also increased by cancer-associated fibroblasts (CAFs). Comparative analysis of matched colorectal cancer specimens from patients before and after cytotoxic treatment revealed a significant increase in CAFs. Chemotherapy-treated human CAFs promoted CIC self-renewal and in vivo tumor growth associated with increased secretion of specific cytokines and chemokines, including interleukin-17A (IL-17A). Exogenous IL-17A increased CIC self-renewal and invasion, and targeting IL-17A signaling impaired CIC growth. Notably, IL-17A was overexpressed by colorectal CAFs in response to chemotherapy with expression validated directly in patient-derived specimens without culture. These data suggest that chemotherapy induces remodeling of the tumor microenvironment to support the tumor cellular hierarchy through secreted factors. Incorporating simultaneous disruption of CIC mechanisms and interplay with the tumor microenvironment could optimize therapeutic targeting of cancer.
Figures








References
-
- American Cancer Society. 2011. http://www.cancer.org/research/cancerfactsstatistics/colorectal-cancer-f... American Cancer Society. Colorectal Cancer Facts & Figures 2011-2013.
-
- Barcellos-Hoff M.H., Ravani S.A. 2000. Irradiated mammary gland stroma promotes the expression of tumorigenic potential by unirradiated epithelial cells. Cancer Res. 60:1254–1260 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K99 CA157948/CA/NCI NIH HHS/United States
- CA154130,/CA/NCI NIH HHS/United States
- NS063057/NS/NINDS NIH HHS/United States
- CA151522/CA/NCI NIH HHS/United States
- R00 CA157948/CA/NCI NIH HHS/United States
- K99/R00 CA157948/CA/NCI NIH HHS/United States
- F30 CA165857/CA/NCI NIH HHS/United States
- CA129958/CA/NCI NIH HHS/United States
- R01 CA151522/CA/NCI NIH HHS/United States
- R21 NS063057/NS/NINDS NIH HHS/United States
- R01 CA129958/CA/NCI NIH HHS/United States
- T32 GM007250/GM/NIGMS NIH HHS/United States
- R01 CA154130/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

