A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis
- PMID: 24323357
- PMCID: PMC3865470
- DOI: 10.1084/jem.20130351
A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis
Abstract
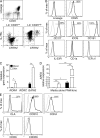
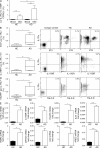
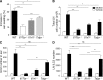
Type 2 innate lymphoid cells (ILC2s, nuocytes, NHC) require RORA and GATA3 for their development. We show that human ILC2s express skin homing receptors and infiltrate the skin after allergen challenge, where they produce the type 2 cytokines IL-5 and IL-13. Skin-derived ILC2s express the IL-33 receptor ST2, which is up-regulated during activation, and are enriched in lesional skin biopsies from atopic patients. Signaling via IL-33 induces type 2 cytokine and amphiregulin expression, and increases ILC2 migration. Furthermore, we demonstrate that E-cadherin ligation on human ILC2 dramatically inhibits IL-5 and IL-13 production. Interestingly, down-regulation of E-cadherin is characteristic of filaggrin insufficiency, a cardinal feature of atopic dermatitis (AD). ILC2 may contribute to increases in type 2 cytokine production in the absence of the suppressive E-cadherin ligation through this novel mechanism of barrier sensing. Using Rag1(-/-) and RORα-deficient mice, we confirm that ILC2s are present in mouse skin and promote AD-like inflammation. IL-25 and IL-33 are the predominant ILC2-inducing cytokines in this model. The presence of ILC2s in skin, and their production of type 2 cytokines in response to IL-33, identifies a role for ILC2s in the pathogenesis of cutaneous atopic disease.
Figures




References
-
- Barlow J.L., Bellosi A., Hardman C.S., Drynan L.F., Wong S.H., Cruickshank J.P., McKenzie A.N. 2012. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J. Allergy Clin. Immunol. 129:191–198: e1–e4 10.1016/j.jaci.2011.09.041 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

