Neutrophils are needed for an effective immune response against pulmonary rat coronavirus infection, but also contribute to pathology
- PMID: 24323639
- PMCID: PMC4093780
- DOI: 10.1099/vir.0.061986-0
Neutrophils are needed for an effective immune response against pulmonary rat coronavirus infection, but also contribute to pathology
Abstract
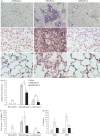
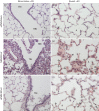
Polymorphonuclear neutrophils (PMN) infiltrate the respiratory tract early after viral infection and can contribute to both host defence and pathology. Coronaviruses are important causes of respiratory tract infections, ranging from mild to severe depending on the viral strain. This study evaluated the role of PMN during a non-fatal pulmonary coronavirus infection in the natural host. Rat coronavirus (RCoV) causes respiratory disease in adult rats, characterized by an early PMN response, viral replication and inflammatory lesions in the lungs, mild weight loss and effective resolution of infection. To determine their role during RCoV infection, PMN were depleted and the effects on disease progression, viral replication, inflammatory response and lung pathology were analysed. Compared with RCoV infection in control animals, PMN-depleted rats had worsened disease with weight loss, clinical signs, mortality and prolonged pulmonary viral replication. PMN-depleted animals had fewer macrophages and lymphocytes in the respiratory tract, corresponding to lower chemokine levels. Combined with in vitro experiments showing that PMN express cytokines and chemokines in response to RCoV-infected alveolar epithelial cells, these findings support a role for PMN in eliciting an inflammatory response to RCoV infection. Despite their critical role in the protection from severe disease, the presence of PMN was correlated with haemorrhagic lesions, epithelial barrier permeability and cellular inflammation in the lungs. This study demonstrated that while PMN are required for an effective antiviral response, they also contribute to lung pathology during RCoV infection.
Figures




References
-
- Assiri A., Al-Tawfiq J. A., Al-Rabeeah A. A., Al-Rabiah F. A., Al-Hajjar S., Al-Barrak A., Flemban H., Al-Nassir W. N., Balkhy H. H. & other authors (2013). Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis 13, 752–761 10.1016/S1473-3099(13)70204-4 - DOI - PMC - PubMed
-
- Cervantes-Barragán L., Kalinke U., Züst R., König M., Reizis B., López-Macías C., Thiel V., Ludewig B. (2009). Type I IFN-mediated protection of macrophages and dendritic cells secures control of murine coronavirus infection. J Immunol 182, 1099–1106 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

