European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: summary of the 2011 4th European Conference on Infections in Leukemia
- PMID: 24323983
- PMCID: PMC3856957
- DOI: 10.3324/haematol.2013.091025
European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: summary of the 2011 4th European Conference on Infections in Leukemia
Erratum in
- Haematologica. 2014 Feb;99(2):400
Abstract
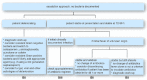
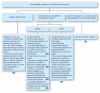
Owing to increasing resistance and the limited arsenal of new antibiotics, especially against Gram-negative pathogens, carefully designed antibiotic regimens are obligatory for febrile neutropenic patients, along with effective infection control. The Expert Group of the 4(th) European Conference on Infections in Leukemia has developed guidelines for initial empirical therapy in febrile neutropenic patients, based on: i) the local resistance epidemiology; and ii) the patient's risk factors for resistant bacteria and for a complicated clinical course. An 'escalation' approach, avoiding empirical carbapenems and combinations, should be employed in patients without particular risk factors. A 'de-escalation' approach, with initial broad-spectrum antibiotics or combinations, should be used only in those patients with: i) known prior colonization or infection with resistant pathogens; or ii) complicated presentation; or iii) in centers where resistant pathogens are prevalent at the onset of febrile neutropenia. In the latter case, infection control and antibiotic stewardship also need urgent review. Modification of the initial regimen at 72-96 h should be based on the patient's clinical course and the microbiological results. Discontinuation of antibiotics after 72 h or later should be considered in neutropenic patients with fever of unknown origin who are hemodynamically stable since presentation and afebrile for at least 48 h, irrespective of neutrophil count and expected duration of neutropenia. This strategy aims to minimize the collateral damage associated with antibiotic overuse, and the further selection of resistance.
Figures
References
-
- Almyroudis NG, Fuller A, Jakubowski A, Sepkowitz K, Jaffe D, Small TN, et al. Pre-and post-engraftment bloodstream infection rates and associated mortality in allogeneic hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2005;7(1): 11–7 - PubMed
-
- Collin BA, Leather HL, Wingard JR, Ramphal R. Evolution, incidence, and susceptibility of bacterial bloodstream isolates from 519 bone marrow transplant patients. Clin Infect Dis. 2001;33(7): 947–53 - PubMed
-
- Mikulska M, Del Bono V, Raiola AM, Bruno B, Gualandi F, Occhini D, et al. Blood stream infections in allogeneic hematopoietic stem cell transplant recipients: reemergence of Gram-negative rods and increasing antibiotic resistance. Biol Blood Marrow Transplant. 2009;15(1): 47–53 - PubMed
-
- Ninin E, Milpied N, Moreau P, Andre-Richet B, Morineau N, Mahe B, et al. Longitudinal study of bacterial, viral, and fungal infections in adult recipients of bone marrow transplants. Clin Infect Dis. 2001; 33(1): 41–7 - PubMed
-
- Poutsiaka DD, Price LL, Ucuzian A, Chan GW, Miller KB, Snydman DR. Blood stream infection after hematopoietic stem cell transplantation is associated with increased mortality. Bone Marrow Transplant. 2007; 40(1): 63–70 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical