Excess LIGHT contributes to placental impairment, increased secretion of vasoactive factors, hypertension, and proteinuria in preeclampsia
- PMID: 24324043
- PMCID: PMC4053533
- DOI: 10.1161/HYPERTENSIONAHA.113.02458
Excess LIGHT contributes to placental impairment, increased secretion of vasoactive factors, hypertension, and proteinuria in preeclampsia
Abstract
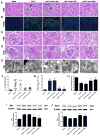
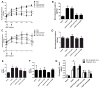
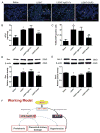
Preeclampsia, a prevalent hypertensive disorder of pregnancy, is believed to be secondary to uteroplacental ischemia. Accumulating evidence indicates that hypoxia-independent mediators, including inflammatory cytokines and growth factors, are associated with preeclampsia, but it is unclear whether these signals directly contribute to placental damage and disease development in vivo. We report that LIGHT, a novel tumor necrosis factor superfamily member, is significantly elevated in the circulation and placentas of preeclamptic women compared with normotensive pregnant women. Injection of LIGHT into pregnant mice induced placental apoptosis, small fetuses, and key features of preeclampsia, hypertension and proteinuria. Mechanistically, using neutralizing antibodies specific for LIGHT receptors, we found that LIGHT receptors herpes virus entry mediator and lymphotoxin β receptor are required for LIGHT-induced placental impairment, small fetuses, and preeclampsia features in pregnant mice. Accordingly, we further revealed that LIGHT functions through these 2 receptors to induce secretion of soluble fms-like tyrosine kinase-1 and endothelin-1, 2 well-accepted pathogenic factors in preeclampsia, and thereby plays an important role in hypertension and proteinuria in pregnant mice. Lastly, we extended our animal findings to human studies and demonstrated that activation of LIGHT receptors resulted in increased apoptosis and elevation of soluble fms-like tyrosine kinase-1 secretion in human placental villous explants. Overall, our human and mouse studies show that LIGHT signaling is a previously unrecognized pathway responsible for placental apoptosis, elevated secretion of vasoactive factors, and subsequent maternal features of preeclampsia, and reveal new therapeutic opportunities for the management of the disease.
Keywords: endothelin-1; herpes virus entry mediator; lymphotoxin-beta receptor; preeclampsia; receptors, tumor necrosis factor, member 14; tumor necrosis factor ligand superfamily member 14.
Figures

References
-
- Roberts JM, Cooper DW. Pathogenesis and genetics of pre-eclampsia. Lancet. 2001;357:53–56. - PubMed
-
- Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of preeclampsia: Linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation. 2002;9:147–160. - PubMed
-
- Godfrey KM, Barker DJ. Fetal nutrition and adult disease. The American journal of clinical nutrition. 2000;71:1344S–1352S. - PubMed
-
- Barker DJ. In utero programming of chronic disease. Clin Sci (Lond) 1998;95:115–128. - PubMed
-
- Saito S, Sakai M. Th1/th2 balance in preeclampsia. Journal of reproductive immunology. 2003;59:161–173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

