Biased migration of confined neutrophil-like cells in asymmetric hydraulic environments
- PMID: 24324148
- PMCID: PMC3876268
- DOI: 10.1073/pnas.1317441110
Biased migration of confined neutrophil-like cells in asymmetric hydraulic environments
Abstract
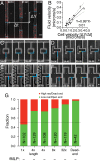
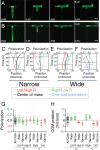
Cells integrate multiple measurement modalities to navigate their environment. Soluble and substrate-bound chemical gradients and physical cues have all been shown to influence cell orientation and migration. Here we investigate the role of asymmetric hydraulic pressure in directional sensing. Cells confined in microchannels identified and chose a path of lower hydraulic resistance in the absence of chemical cues. In a bifurcating channel with asymmetric hydraulic resistances, this choice was preceded by the elaboration of two leading edges with a faster extension rate along the lower resistance channel. Retraction of the "losing" edge appeared to precipitate a final choice of direction. The pressure differences altering leading edge protrusion rates were small, suggesting weak force generation by leading edges. The response to the physical asymmetry was able to override a dynamically generated chemical cue. Motile cells may use this bias as a result of hydraulic resistance, or "barotaxis," in concert with chemotaxis to navigate complex environments.
Keywords: cell confinement; cell migration; microfluidics; photocaged chemoattractant; physical forces.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Insall RH. Understanding eukaryotic chemotaxis: A pseudopod-centred view. Nat Rev Mol Cell Biol. 2010;11(6):453–458. - PubMed
-
- Parent CA. Making all the right moves: Chemotaxis in neutrophils and Dictyostelium. Curr Opin Cell Biol. 2004;16(1):4–13. - PubMed
-
- Kay RR, Langridge P, Traynor D, Hoeller O. Changing directions in the study of chemotaxis. Nat Rev Mol Cell Biol. 2008;9(6):455–463. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

