The adjuvant MF59 induces ATP release from muscle that potentiates response to vaccination
- PMID: 24324152
- PMCID: PMC3876261
- DOI: 10.1073/pnas.1319784110
The adjuvant MF59 induces ATP release from muscle that potentiates response to vaccination
Abstract
Vaccines are the most effective agents to control infections. In addition to the pathogen antigens, vaccines contain adjuvants that are used to enhance protective immune responses. However, the molecular mechanism of action of most adjuvants is ill-known, and a better understanding of adjuvanticity is needed to develop improved adjuvants based on molecular targets that further enhance vaccine efficacy. This is particularly important for tuberculosis, malaria, AIDS, and other diseases for which protective vaccines do not exist. Release of endogenous danger signals has been linked to adjuvanticity; however, the role of extracellular ATP during vaccination has never been explored. Here, we tested whether ATP release is involved in the immune boosting effect of four common adjuvants: aluminum hydroxide, calcium phosphate, incomplete Freund's adjuvant, and the oil-in-water emulsion MF59. We found that intramuscular injection is always associated with a weak transient release of ATP, which was greatly enhanced by the presence of MF59 but not by all other adjuvants tested. Local injection of apyrase, an ATP-hydrolyzing enzyme, inhibited cell recruitment in the muscle induced by MF59 but not by alum or incomplete Freund's adjuvant. In addition, apyrase strongly inhibited influenza-specific T-cell responses and hemagglutination inhibition titers in response to an MF59-adjuvanted trivalent influenza vaccine. These data demonstrate that a transient ATP release is required for innate and adaptive immune responses induced by MF59 and link extracellular ATP with an enhanced response to vaccination.
Keywords: DAMP; danger associated molecular pattern; inflammation; vaccine adjuvants.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

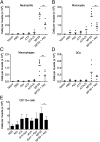
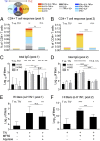

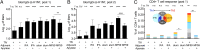
References
-
- McKee AS, Munks MW, Marrack P. How do adjuvants work? Important considerations for new generation adjuvants. Immunity. 2007;27(5):687–690. - PubMed
-
- Lambrecht BN, Kool M, Willart MA, Hammad H. Mechanism of action of clinically approved adjuvants. Curr Opin Immunol. 2009;21(1):23–29. - PubMed
-
- Tritto E, Mosca F, De Gregorio E. Mechanism of action of licensed vaccine adjuvants. Vaccine. 2009;27(25-26):3331–3334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

