Genetic disruption of CD8+ Treg activity enhances the immune response to viral infection
- PMID: 24324159
- PMCID: PMC3876206
- DOI: 10.1073/pnas.1320999110
Genetic disruption of CD8+ Treg activity enhances the immune response to viral infection
Abstract
The immunological interactions that regulate the T-cell response to chronic viral infection are insufficiently understood. Here we study a cellular interaction that may enhance the antiviral immune response and constrain immunopathology. We analyze the contribution of Qa-1-restricted CD8(+) regulatory T cells (Treg cells) to antiviral immunity after infection by lymphocytic choriomeningitis virus. These CD8(+) Treg cells recognize and eliminate target cells through an interaction with the murine class Ib MHC molecule Qa-1 (HLA-E in humans). Using Qa-1 mutant mice (B6.Qa-1-D227K [B6-DK]) that harbor a single mutation that abrogates binding of Qa-1 peptide to the CD8-TCR (T-cell receptor) complex, we show that disruption of immune suppression mediated by CD8(+) Treg cells results in robust antiviral immune responses in both acute and chronic viral infection. Enhanced antiviral responses of B6-DK mice were accompanied by increased control of virus, reduced tissue inflammation in the acute phase, and dramatic alleviation of disease in the chronic phase. In addition, CD8(+) effector T cells in B6-DK mice displayed a less exhausted phenotype characterized by decreased expression of programmed cell death 1 (PD-1), LAG3 (CD223), and 2B4 (CD244) and increased expression of NKG2D (CD314) and killer cell lectin-like receptor subfamily G member 1 (KLRG1). Enhanced antiviral immunity in B6-DK mice reflected, in part, reduced inhibition of CD8(+) effector cells by CD8(+) Treg cells. These findings indicate that direct inhibition of effector CD8(+) T cells by Qa-1-restricted CD8(+) Treg cells results in increased disease severity and delayed recovery. These data suggest that depletion or inactivation of CD8(+) Treg cells represents a potentially effective strategy to enhance protective immunity to chronic viral infection.
Keywords: T-cell exhaustion; immune regulation; killer cell Ig-like receptor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



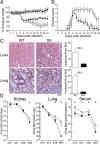
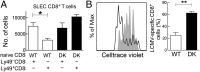
References
-
- Jost S, Altfeld M. Control of human viral infections by natural killer cells. Annu Rev Immunol. 2013;31:163–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

