Small molecule-mediated directed differentiation of human embryonic stem cells toward ventricular cardiomyocytes
- PMID: 24324277
- PMCID: PMC3902291
- DOI: 10.5966/sctm.2013-0110
Small molecule-mediated directed differentiation of human embryonic stem cells toward ventricular cardiomyocytes
Abstract
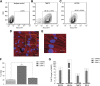
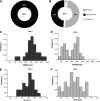
The generation of human ventricular cardiomyocytes from human embryonic stem cells and/or induced pluripotent stem cells could fulfill the demand for therapeutic applications and in vitro pharmacological research; however, the production of a homogeneous population of ventricular cardiomyocytes remains a major limitation. By combining small molecules and growth factors, we developed a fully chemically defined, directed differentiation system to generate ventricular-like cardiomyocytes (VCMs) from human embryonic stem cells and induced pluripotent stem cells with high efficiency and reproducibility. Molecular characterization revealed that the differentiation recapitulated the developmental steps of cardiovascular fate specification. Electrophysiological analyses further illustrated the generation of a highly enriched population of VCMs. These chemically induced VCMs exhibited the expected cardiac electrophysiological and calcium handling properties as well as the appropriate chronotropic responses to cardioactive compounds. In addition, using an integrated computational and experimental systems biology approach, we demonstrated that the modulation of the canonical Wnt pathway by the small molecule IWR-1 plays a key role in cardiomyocyte subtype specification. In summary, we developed a reproducible and efficient experimental platform that facilitates a chemical genetics-based interrogation of signaling pathways during cardiogenesis that bypasses the limitations of genetic approaches and provides a valuable source of ventricular cardiomyocytes for pharmacological screenings as well as cell replacement therapies.
Keywords: Cardiac; Differentiation; Embryonic stem cells; Pluripotent stem cells.
Figures





References
-
- Noseda M, Peterkin T, Simões FC, et al. Cardiopoietic factors: Extracellular signals for cardiac lineage commitment. Circ Res. 2011;108:129–152. - PubMed
-
- Laflamme MA, Chen KY, Naumova AV, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

