PD-L1 blockade attenuated sepsis-induced liver injury in a mouse cecal ligation and puncture model
- PMID: 24324295
- PMCID: PMC3844221
- DOI: 10.1155/2013/361501
PD-L1 blockade attenuated sepsis-induced liver injury in a mouse cecal ligation and puncture model
Abstract
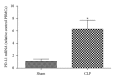
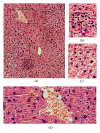
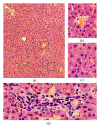
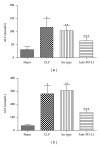
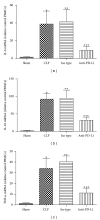
Liver plays a major role in hypermetabolism and produces acute phase proteins during systemic inflammatory response syndrome and it is of vital importance in host defense and bacteria clearance. Our previous studies indicated that programmed death-1 (PD-1) and its ligand programmed death ligand-1 (PD-L1) are crucial modulators of host immune responses during sepsis. Our current study was designed to investigate the role of PD-L1 in sepsis-induced liver injury by a mouse cecal ligation and puncture (CLP) model. Our results indicated that there was a significant increase of PD-L1 expression in liver after CLP challenge compared to sham-operated controls, in terms of levels of mRNA transcription and immunohistochemistry. Anti-PD-L1 antibody significantly alleviated the morphology of liver injury in CLP mice. Anti-PD-L1 antibody administration decreased ALT and AST release in CLP mice, decreased the levels of tumor necrosis factor (TNF)-α , interleukin (IL)-6, and IL-10 mRNA in liver after sepsis challenge. Thus, anti-PD-L1 antibody might have a therapeutic potential in attenuating liver injury in sepsis.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

