Next-generation transgenic mice for optogenetic analysis of neural circuits
- PMID: 24324405
- PMCID: PMC3840435
- DOI: 10.3389/fncir.2013.00160
Next-generation transgenic mice for optogenetic analysis of neural circuits
Abstract
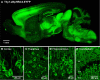
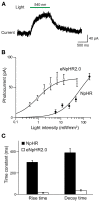
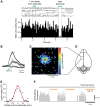
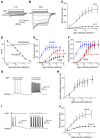
Here we characterize several new lines of transgenic mice useful for optogenetic analysis of brain circuit function. These mice express optogenetic probes, such as enhanced halorhodopsin or several different versions of channelrhodopsins, behind various neuron-specific promoters. These mice permit photoinhibition or photostimulation both in vitro and in vivo. Our results also reveal the important influence of fluorescent tags on optogenetic probe expression and function in transgenic mice.
Keywords: cerebellum; channelrhodopsin; cortex; hippocampus; optogenetics; photoinhibition; photostimulation; pons.
Figures



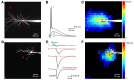
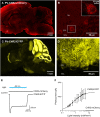

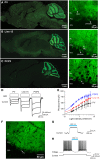
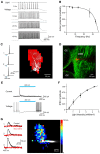
References
-
- Anchisi D., Scelfo B., Tempia F. (2001). Postsynaptic currents in deep cerebellar nuclei. J. Neurophysiol. 85, 323–331 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

