From ear to uncertainty: vestibular contributions to cognitive function
- PMID: 24324413
- PMCID: PMC3840327
- DOI: 10.3389/fnint.2013.00084
From ear to uncertainty: vestibular contributions to cognitive function
Abstract
In addition to the deficits in the vestibulo-ocular and vestibulo-spinal reflexes that occur following vestibular dysfunction, there is substantial evidence that vestibular loss also causes cognitive disorders, some of which may be due to the reflexive deficits and some of which are related to the role that ascending vestibular pathways to the limbic system and neocortex play in spatial orientation. In this review we summarize the evidence that vestibular loss causes cognitive disorders, especially spatial memory deficits, in animals and humans and critically evaluate the evidence that these deficits are not due to hearing loss, problems with motor control, oscillopsia or anxiety and depression. We review the evidence that vestibular lesions affect head direction and place cells as well as the emerging evidence that artificial activation of the vestibular system, using galvanic vestibular stimulation (GVS), can modulate cognitive function.
Keywords: cognition; hippocampus; spatial memory; vestibular; vestibular lesions.
Figures
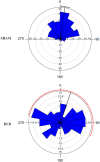
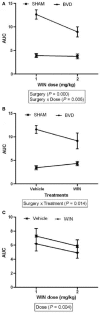
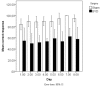

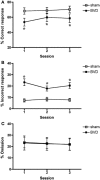




References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

