Effect of prenatal cocaine on early postnatal thermoregulation and ultrasonic vocalization production
- PMID: 24324452
- PMCID: PMC3840503
- DOI: 10.3389/fpsyg.2013.00882
Effect of prenatal cocaine on early postnatal thermoregulation and ultrasonic vocalization production
Abstract
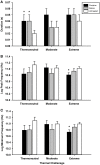
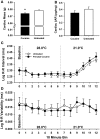
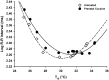
Prenatal cocaine exposure can alter the postnatal care received by rat pups. Such effects could be caused in part by alterations in pup-produced stimuli that elicit early postnatal maternal care. Pup ultrasonic vocalizations are thought to be a particularly salient stimulus, and when paired with other cues, may elicit maternal attention. Cocaine is known to acutely alter thermoregulatory and cardiac function, thus prenatal cocaine may affect vocalizations through altering these functions. The data presented here determine the impact of full term prenatal cocaine exposure, saline exposure, or no exposure on thermogenic capacity, cardiac function, and the resulting ultrasonic vocalizations across the early postnatal period (days 1-5). Results indicated that while sharing many similar characteristics with saline-exposed and untreated animals, prenatal cocaine exposure was associated with specific alterations in vocalization characteristics on postnatal day 1 (PND 1), including call amplitude. Furthermore, numerous spectral parameters of their vocalizations were found altered on PND 3, including rate, call duration, and frequency, while no alterations were found on PND 5. Additionally, cocaine-exposed pups also showed a reduced thermoregulatory capacity compared to saline animals and reduced cardiac mass compared to untreated animals on PND 5. Together, these findings indicate that prenatal cocaine may be altering the elicitation of maternal care through its impact on vocalizations and thermoregulation, and suggests a potential mechanism for these effects through cocaine's impact on developing stress systems.
Keywords: brown adipose tissue; cardiac; prenatal cocaine; stress; thermogenesis; ultrasonic vocalization.
Figures






Similar articles
-
Early postpartum pup preference is altered by gestational cocaine treatment: associations with infant cues and oxytocin expression in the MPOA.Behav Brain Res. 2015 Feb 1;278:176-85. doi: 10.1016/j.bbr.2014.09.045. Epub 2014 Oct 6. Behav Brain Res. 2015. PMID: 25300467 Free PMC article.
-
Translational analysis of effects of prenatal cocaine exposure on human infant cries and rat pup ultrasonic vocalizations.PLoS One. 2014 Oct 22;9(10):e110349. doi: 10.1371/journal.pone.0110349. eCollection 2014. PLoS One. 2014. PMID: 25338015 Free PMC article.
-
Effects of prenatal cocaine on behavioral responses to a cocaine challenge on postnatal day 11.Neurotoxicol Teratol. 1992 May-Jun;14(3):183-9. doi: 10.1016/0892-0362(92)90014-2. Neurotoxicol Teratol. 1992. PMID: 1635539
-
Prenatal cocaine produces biochemical and functional changes in brain serotonin systems in rat progeny.NIDA Res Monogr. 1995;158:115-48. NIDA Res Monogr. 1995. PMID: 8594482 Review.
-
Effects of prenatal morphine and cocaine on postnatal behaviors and brain neurotransmitters.NIDA Res Monogr. 1995;158:88-114. NIDA Res Monogr. 1995. PMID: 8594491 Review.
Cited by
-
Pharmacology of Ultrasonic Vocalizations in adult Rats: Significance, Call Classification and Neural Substrate.Curr Neuropharmacol. 2015;13(2):180-92. doi: 10.2174/1570159x13999150210141444. Curr Neuropharmacol. 2015. PMID: 26411761 Free PMC article. Review.
-
Acoustic complexity of pup isolation calls in Mongolian hamsters: 3-frequency phenomena and chaos.Curr Zool. 2023 Jul 24;70(5):559-574. doi: 10.1093/cz/zoad036. eCollection 2024 Oct. Curr Zool. 2023. PMID: 39463689 Free PMC article.
-
Early postpartum pup preference is altered by gestational cocaine treatment: associations with infant cues and oxytocin expression in the MPOA.Behav Brain Res. 2015 Feb 1;278:176-85. doi: 10.1016/j.bbr.2014.09.045. Epub 2014 Oct 6. Behav Brain Res. 2015. PMID: 25300467 Free PMC article.
-
The Effects of Perinatal Oxycodone Exposure on Behavioral Outcome in a Rodent Model.Front Pediatr. 2017 Aug 25;5:180. doi: 10.3389/fped.2017.00180. eCollection 2017. Front Pediatr. 2017. PMID: 28971091 Free PMC article.
-
Translational analysis of effects of prenatal cocaine exposure on human infant cries and rat pup ultrasonic vocalizations.PLoS One. 2014 Oct 22;9(10):e110349. doi: 10.1371/journal.pone.0110349. eCollection 2014. PLoS One. 2014. PMID: 25338015 Free PMC article.
References
-
- Antonelli T., Tomasini M. C., Tattoli M., Cassano T., Tanganelli S., Finetti S., et al. (2005). Prenatal exposure to the CB1 receptor agonist WIN 55,212-2 causes learning disruption associated with impaired cortical NMDA receptor function and emotional reactivity changes in rat offspring. Cereb. cortex 15, 2013–2020 10.1093/cercor/bhi076 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

