Small-molecule theranostic probes: a promising future in neurodegenerative diseases
- PMID: 24324497
- PMCID: PMC3845517
- DOI: 10.1155/2013/150952
Small-molecule theranostic probes: a promising future in neurodegenerative diseases
Abstract
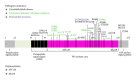
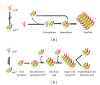
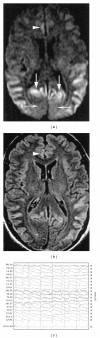
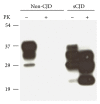
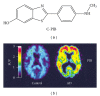
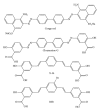
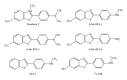
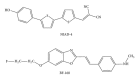
Prion diseases are fatal neurodegenerative illnesses, which include Creutzfeldt-Jakob disease in humans and scrapie, chronic wasting disease, and bovine spongiform encephalopathy in animals. They are caused by unconventional infectious agents consisting primarily of misfolded, aggregated, β -sheet-rich isoforms, denoted prions, of the physiological cellular prion protein (PrP(C)). Many lines of evidence suggest that prions (PrP(Sc)) act both as a template for this conversion and as a neurotoxic agent causing neuronal dysfunction and cell death. As such, PrP(Sc) may be considered as both a neuropathological hallmark of the disease and a therapeutic target. Several diagnostic imaging probes have been developed to monitor cerebral amyloid lesions in patients with neurodegenerative disorders (such as Alzheimer's disease, Parkinson's disease, and prion disease). Examples of these probes are Congo red, thioflavin T, and their derivatives. We synthesized a series of styryl derivatives, denoted theranostics, and studied their therapeutic and/or diagnostic potentials. Here we review the salient traits of these small molecules that are able to detect and modulate aggregated forms of several proteins involved in protein misfolding diseases. We then highlight the importance of further studies for their practical implications in therapy and diagnostics.
Figures

References
-
- Rowe CC, Ackerman U, Browne W, et al. Imaging of amyloid β in Alzheimer’s disease with 18F-BAY94-9172, a novel PET tracer: proof of mechanism. The Lancet Neurology. 2008;7(2):129–135. - PubMed
-
- O'keefe GJ, Saunder TH, Ng S, et al. Radiation dosimetry of beta-amyloid tracers 11c-Pib and 18f-bay94-9172. Journal of Nuclear Medicine. 50:309–315. - PubMed
-
- Koole M, Lewis DM, Buckley C, et al. Whole-body biodistribution and radiation dosimetry of 18F-GE067: a radioligand for in vivo brain amyloid imaging. Journal of Nuclear Medicine. 2009;50(5):818–822. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

