Canonical Wnt signaling promotes early hematopoietic progenitor formation and erythroid specification during embryonic stem cell differentiation
- PMID: 24324557
- PMCID: PMC3850021
- DOI: 10.1371/journal.pone.0081030
Canonical Wnt signaling promotes early hematopoietic progenitor formation and erythroid specification during embryonic stem cell differentiation
Abstract
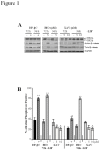
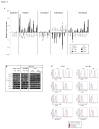
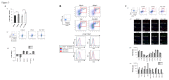
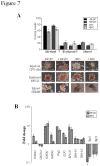
The generation of hematopoietic stem cells (HSCs) during development is a complex process linked to morphogenic signals. Understanding this process is important for regenerative medicine applications that require in vitro production of HSC. In this study we investigated the effects of canonical Wnt/β-catenin signaling during early embryonic differentiation and hematopoietic specification using an embryonic stem cell system. Our data clearly demonstrates that following early differentiation induction, canonical Wnt signaling induces a strong mesodermal program whilst maintaining a degree of stemness potential. This involved a complex interplay between β-catenin/TCF/LEF/Brachyury/Nanog. β-catenin mediated up-regulation of TCF/LEF resulted in enhanced brachyury levels, which in-turn lead to Nanog up-regulation. During differentiation, active canonical Wnt signaling also up-regulated key transcription factors and cell specific markers essential for hematopoietic specification, in particular genes involved in establishing primitive erythropoiesis. This led to a significant increase in primitive erythroid colony formation. β-catenin signaling also augmented early hematopoietic and multipotent progenitor (MPP) formation. Following culture in a MPP specific cytokine cocktail, activation of β-catenin suppressed differentiation of the early hematopoietic progenitor population, with cells displaying a higher replating capacity and a propensity to form megakaryocytic erythroid progenitors. This bias towards erythroid lineage commitment was also observed when hematopoietic progenitors were directed to undergo myeloid colony formation. Overall this study underscores the importance of canonical Wnt/β-catenin signaling in mesodermal specification, primitive erythropoiesis and early hematopietic progenitor formation during hematopoietic induction.
Conflict of interest statement
Figures



Similar articles
-
The negative impact of Wnt signaling on megakaryocyte and primitive erythroid progenitors derived from human embryonic stem cells.Stem Cell Res. 2014 Mar;12(2):441-51. doi: 10.1016/j.scr.2013.12.003. Epub 2013 Dec 16. Stem Cell Res. 2014. PMID: 24412757 Free PMC article.
-
Cooperation between both Wnt/{beta}-catenin and PTEN/PI3K/Akt signaling promotes primitive hematopoietic stem cell self-renewal and expansion.Genes Dev. 2011 Sep 15;25(18):1928-42. doi: 10.1101/gad.17421911. Epub 2011 Sep 2. Genes Dev. 2011. PMID: 21890648 Free PMC article.
-
Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block.Nat Immunol. 2006 Oct;7(10):1048-56. doi: 10.1038/ni1381. Epub 2006 Sep 3. Nat Immunol. 2006. PMID: 16951689
-
Maintaining embryonic stem cell pluripotency with Wnt signaling.Development. 2011 Oct;138(20):4341-50. doi: 10.1242/dev.066209. Epub 2011 Sep 8. Development. 2011. PMID: 21903672 Free PMC article. Review.
-
Wnt/β-catenin signaling in embryonic stem cell self-renewal and somatic cell reprogramming.Stem Cell Rev Rep. 2011 Nov;7(4):836-46. doi: 10.1007/s12015-011-9275-1. Stem Cell Rev Rep. 2011. PMID: 21603945 Review.
Cited by
-
NKL homeobox gene activities in normal and malignant myeloid cells.PLoS One. 2019 Dec 11;14(12):e0226212. doi: 10.1371/journal.pone.0226212. eCollection 2019. PLoS One. 2019. PMID: 31825998 Free PMC article.
-
Sensitive Quantitative Proteomics of Human Hematopoietic Stem and Progenitor Cells by Data-independent Acquisition Mass Spectrometry.Mol Cell Proteomics. 2019 Jul;18(7):1454-1467. doi: 10.1074/mcp.TIR119.001431. Epub 2019 Apr 11. Mol Cell Proteomics. 2019. PMID: 30975897 Free PMC article.
-
Canonical Wnt: a safeguard and threat for erythropoiesis.Blood Adv. 2021 Sep 28;5(18):3726-3735. doi: 10.1182/bloodadvances.2021004845. Blood Adv. 2021. PMID: 34516644 Free PMC article. Review.
-
Loss of Wnt4 expression inhibits the odontogenic potential of dental pulp stem cells through JNK signaling in pulpitis.Am J Transl Res. 2019 Mar 15;11(3):1819-1826. eCollection 2019. Am J Transl Res. 2019. PMID: 30972205 Free PMC article.
-
GATA2 regulates Wnt signaling to promote primitive red blood cell fate.Dev Biol. 2015 Nov 1;407(1):1-11. doi: 10.1016/j.ydbio.2015.08.012. Epub 2015 Sep 10. Dev Biol. 2015. PMID: 26365900 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

