Rb suppresses collective invasion, circulation and metastasis of breast cancer cells in CD44-dependent manner
- PMID: 24324613
- PMCID: PMC3851742
- DOI: 10.1371/journal.pone.0080590
Rb suppresses collective invasion, circulation and metastasis of breast cancer cells in CD44-dependent manner
Abstract
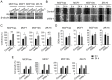
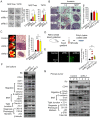
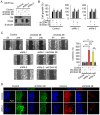
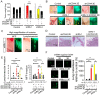
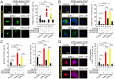
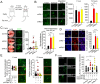
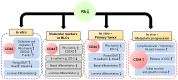
Basal-like breast carcinomas (BLCs) present with extratumoral lymphovascular invasion, are highly metastatic, presumably through a hematogenous route, have augmented expression of CD44 oncoprotein and relatively low levels of retinoblastoma (Rb) tumor suppressor. However, the causal relation among these features is not clear. Here, we show that Rb acts as a key suppressor of multiple stages of metastatic progression. Firstly, Rb suppresses collective cell migration (CCM) and CD44-dependent formation of F-actin positive protrusions in vitro and cell-cluster based lymphovascular invasion in vivo. Secondly, Rb inhibits the release of single cancer cells and cell clusters into the hematogenous circulation and subsequent metastatic growth in lungs. Finally, CD44 expression is required for collective motility and all subsequent stages of metastatic progression initiated by loss of Rb function. Altogether, our results suggest that Rb/CD44 pathway is a crucial regulator of CCM and metastatic progression of BLCs and a promising target for anti-BLCs therapy.
Conflict of interest statement
Figures

References
-
- Friedl P, Alexander S (2011) Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 147: 992–1009. - PubMed
-
- Sabatier R, Jacquemier J, Bertucci F, Esterni B, Finetti P, et al. (2011) Peritumoural vascular invasion: a major determinant of triple-negative breast cancer outcome. Eur J Cancer 47: 1537–1545. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

