Mycobacterium tuberculosis specific CD8(+) T cells rapidly decline with antituberculosis treatment
- PMID: 24324704
- PMCID: PMC3852504
- DOI: 10.1371/journal.pone.0081564
Mycobacterium tuberculosis specific CD8(+) T cells rapidly decline with antituberculosis treatment
Abstract
Rationale: Biomarkers associated with response to therapy in tuberculosis could have broad clinical utility. We postulated that the frequency of Mycobacterium tuberculosis (Mtb) specific CD8(+) T cells, by virtue of detecting intracellular infection, could be a surrogate marker of response to therapy and would decrease during effective antituberculosis treatment.
Objectives: We sought to determine the relationship of Mtb specific CD4(+) T cells and CD8(+) T cells with duration of antituberculosis treatment.
Materials and methods: We performed a prospective cohort study, enrolling between June 2008 and August 2010, of HIV-uninfected Ugandan adults (n = 50) with acid-fast bacillus smear-positive, culture confirmed pulmonary TB at the onset of antituberculosis treatment and the Mtb specific CD4(+) and CD8(+) T cell responses to ESAT-6 and CFP-10 were measured by IFN-γ ELISPOT at enrollment, week 8 and 24.
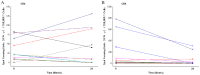
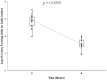
Results: There was a significant difference in the Mtb specific CD8(+) T response, but not the CD4(+) T cell response, over 24 weeks of antituberculosis treatment (p<0.0001), with an early difference observed at 8 weeks of therapy (p = 0.023). At 24 weeks, the estimated Mtb specific CD8(+) T cell response decreased by 58%. In contrast, there was no significant difference in the Mtb specific CD4(+) T cell during the treatment. The Mtb specific CD4(+) T cell response, but not the CD8(+) response, was negatively impacted by the body mass index.
Conclusions: Our data provide evidence that the Mtb specific CD8(+) T cell response declines with antituberculosis treatment and could be a surrogate marker of response to therapy. Additional research is needed to determine if the Mtb specific CD8(+) T cell response can detect early treatment failure, relapse, or to predict disease progression.
Conflict of interest statement
Figures


References
-
- Wallis RS, Doherty TM, Onyebujoh P, Vahedi M, Laang H, et al. (2009) Biomarkers for tuberculosis disease activity, cure, and relapse. Lancet Infect Dis 9: 162–172. - PubMed
-
- Walzl G, Ronacher K, Djoba Siawaya JF, Dockrell HM (2008) Biomarkers for TB treatment response: challenges and future strategies. J Infect 57: 103–109. - PubMed
-
- Lalvani A (2004) Counting antigen-specific T cells: a new approach for monitoring response to tuberculosis treatment? Clin Infect Dis 38: 757–759. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

