Microarray and morphological analysis of early postnatal CRB2 mutant retinas on a pure C57BL/6J genetic background
- PMID: 24324803
- PMCID: PMC3855766
- DOI: 10.1371/journal.pone.0082532
Microarray and morphological analysis of early postnatal CRB2 mutant retinas on a pure C57BL/6J genetic background
Abstract
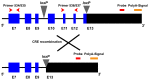
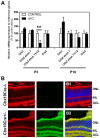
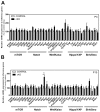
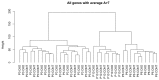
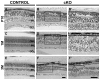
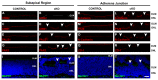
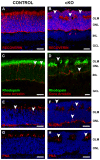
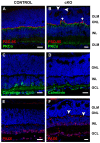
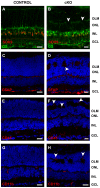
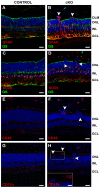
In humans, the Crumbs homologue-1 (CRB1) gene is mutated in progressive types of autosomal recessive retinitis pigmentosa and Leber congenital amaurosis. The severity of the phenotype due to human CRB1 or mouse Crb1 mutations is dependent on the genetic background. Mice on C57BL/6J background with Crb1 mutations show late onset of retinal spotting phenotype or no phenotype. Recently, we showed that conditional deletion of mouse Crb2 in the retina results in early retinal disorganization leading to severe and progressive retinal degeneration with concomitant visual loss that mimics retinitis pigmentosa due to mutations in the CRB1 gene. Recent studies in the fruit fly and zebrafish suggest roles of the Crumbs (CRB) complex members in the regulation of cellular signalling pathways including the Notch1, mechanistic target of rapamycin complex 1 (mTORC1) and the Hippo pathway. Here, we demonstrate that mice backcrossed to C57BL/6J background with loss of CRB2 in the retina show a progressive disorganization and degeneration phenotype during late retinal development. We used microarray gene profiling to study the transcriptome of retinas lacking CRB2 during late retinal development. Unexpectedly, the retinas of newborn mice lacking CRB2 showed no changes in the transcriptome during retinal development. These findings suggest that loss of CRB2 in the developing retina results in retinal disorganization and subsequent degeneration without major changes in the transcriptome of the retina. These mice might be an interesting model to study the onset of retinal degeneration upon loss of CRB proteins.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

