Biotransformation of the high-molecular weight polycyclic aromatic hydrocarbon (PAH) benzo[k]fluoranthene by Sphingobium sp. strain KK22 and identification of new products of non-alternant PAH biodegradation by liquid chromatography electrospray ionization tandem mass spectrometry
- PMID: 24325265
- PMCID: PMC3937716
- DOI: 10.1111/1751-7915.12102
Biotransformation of the high-molecular weight polycyclic aromatic hydrocarbon (PAH) benzo[k]fluoranthene by Sphingobium sp. strain KK22 and identification of new products of non-alternant PAH biodegradation by liquid chromatography electrospray ionization tandem mass spectrometry
Abstract
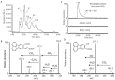
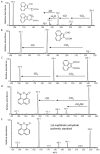
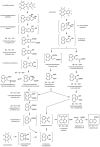
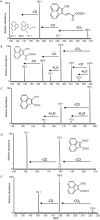
A pathway for the biotransformation of the environmental pollutant and high-molecular weight polycyclic aromatic hydrocarbon (PAH) benzo[k]fluoranthene by a soil bacterium was constructed through analyses of results from liquid chromatography negative electrospray ionization tandem mass spectrometry (LC/ESI(-)-MS/MS). Exposure of Sphingobium sp. strain KK22 to benzo[k]fluoranthene resulted in transformation to four-, three- and two-aromatic ring products. The structurally similar four- and three-ring non-alternant PAHs fluoranthene and acenaphthylene were also biotransformed by strain KK22, and LC/ESI(-)-MS/MS analyses of these products confirmed the lower biotransformation pathway proposed for benzo[k]fluoranthene. In all, seven products from benzo[k]fluoranthene and seven products from fluoranthene were revealed and included previously unreported products from both PAHs. Benzo[k]fluoranthene biotransformation proceeded through ortho-cleavage of 8,9-dihydroxy-benzo[k]fluoranthene to 8-carboxyfluoranthenyl-9-propenic acid and 9-hydroxy-fluoranthene-8-carboxylic acid, and was followed by meta-cleavage to produce 3-(2-formylacenaphthylen-1-yl)-2-hydroxy-prop-2-enoic acid. The fluoranthene pathway converged with the benzo[k]fluoranthene pathway through detection of the three-ring product, 2-formylacenaphthylene-1-carboxylic acid. Production of key downstream metabolites, 1,8-naphthalic anhydride and 1-naphthoic acid from benzo[k]fluoranthene, fluoranthene and acenaphthylene biotransformations provided evidence for a common pathway by strain KK22 for all three PAHs through acenaphthoquinone. Quantitative analysis of benzo[k]fluoranthene biotransformation by strain KK22 confirmed biodegradation. This is the first pathway proposed for the biotransformation of benzo[k]fluoranthene by a bacterium.
© 2013 The Authors. Microbial Biotechnology published by John Wiley & Sons Ltd and Society for Applied Microbiology.
Figures
Similar articles
-
Utilization of the C9 aromatic hydrocarbon n-propylbenzene by Sphingobium barthaii KK22 and coexistence of multiple biodegradation pathways.Biodegradation. 2025 Jun 23;36(4):54. doi: 10.1007/s10532-025-10149-x. Biodegradation. 2025. PMID: 40549172
-
Benz[a]anthracene biotransformation and production of ring fission products by Sphingobium sp. strain KK22.Appl Environ Microbiol. 2013 Jul;79(14):4410-20. doi: 10.1128/AEM.01129-13. Epub 2013 May 17. Appl Environ Microbiol. 2013. PMID: 23686261 Free PMC article.
-
9,10-Phenanthrenedione biodegradation by a soil bacterium and identification of transformation products by LC/ESI-MS/MS.Chemosphere. 2013 Sep;92(11):1442-9. doi: 10.1016/j.chemosphere.2013.03.054. Epub 2013 Apr 20. Chemosphere. 2013. PMID: 23611246
-
Advances in the field of high-molecular-weight polycyclic aromatic hydrocarbon biodegradation by bacteria.Microb Biotechnol. 2010 Mar;3(2):136-64. doi: 10.1111/j.1751-7915.2009.00130.x. Epub 2009 Jun 22. Microb Biotechnol. 2010. PMID: 21255317 Free PMC article. Review.
-
A Systematic Review of Polycyclic Aromatic Hydrocarbon Derivatives: Occurrences, Levels, Biotransformation, Exposure Biomarkers, and Toxicity.Environ Sci Technol. 2023 Oct 17;57(41):15314-15335. doi: 10.1021/acs.est.3c03170. Epub 2023 Sep 13. Environ Sci Technol. 2023. PMID: 37703436 Review.
Cited by
-
Potential Toxicity Risk Assessment and Priority Control Strategy for PAHs Metabolism and Transformation Behaviors in the Environment.Int J Environ Res Public Health. 2022 Sep 2;19(17):10972. doi: 10.3390/ijerph191710972. Int J Environ Res Public Health. 2022. PMID: 36078713 Free PMC article.
-
Periodic and Spatial Spreading of Alkanes and Alcanivorax Bacteria in Deep Waters of the Mariana Trench.Appl Environ Microbiol. 2019 Jan 23;85(3):e02089-18. doi: 10.1128/AEM.02089-18. Print 2019 Feb 1. Appl Environ Microbiol. 2019. PMID: 30446553 Free PMC article.
-
Utilization of the C9 aromatic hydrocarbon n-propylbenzene by Sphingobium barthaii KK22 and coexistence of multiple biodegradation pathways.Biodegradation. 2025 Jun 23;36(4):54. doi: 10.1007/s10532-025-10149-x. Biodegradation. 2025. PMID: 40549172
-
Biofilm and Planktonic Bacterial and Fungal Communities Transforming High-Molecular-Weight Polycyclic Aromatic Hydrocarbons.Appl Environ Microbiol. 2016 Apr 4;82(8):2288-2299. doi: 10.1128/AEM.03713-15. Print 2016 Apr. Appl Environ Microbiol. 2016. PMID: 26850299 Free PMC article.
-
Complete Genome Sequence of Sphingobium barthaii KK22, a High-Molecular-Weight Polycyclic Aromatic Hydrocarbon-Degrading Soil Bacterium.Microbiol Resour Announc. 2021 Jan 7;10(1):e01250-20. doi: 10.1128/MRA.01250-20. Microbiol Resour Announc. 2021. PMID: 33414343 Free PMC article.
References
-
- Achten C, Cheng S, Straub KL, Hofmann T. The lack of microbial degradation of polycyclic aromatic hydrocarbons from coal-rich soils. Environ Pollut. 2011;159:623–629. - PubMed
-
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for Polycyclic Aromatic Hydrocarbons (PAHs) Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service; 1995. - PubMed
-
- Atlas RM. Handbook of Microbiological Media. Boca Raton, FL: CRC Press; 1993.
-
- Baboshin M, Akimov V, Baskunov B, Born TL, Khan SU, Golovleva L. Conversion of polycyclic aromatic hydrocarbons by Sphingomonas sp. VKM B-2434. Biodegradation. 2008;19:567–576. - PubMed
-
- Balashova N, Stolz A, Knackmuss H, Kosheleva I, Naumov A, Boronin A. Purification and characterization of a salicylate hydroxylase involved in 1-hydroxy-2-naphthoic acid hydroxylation from the naphthalene and phenanthrene-degrading bacterial strain Pseudomonas putida BS202-P1. Biodegradation. 2001;12:179–188. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

