Circulating dendritic cell precursors in chronic kidney disease: a cross-sectional study
- PMID: 24325304
- PMCID: PMC3878881
- DOI: 10.1186/1471-2369-14-274
Circulating dendritic cell precursors in chronic kidney disease: a cross-sectional study
Abstract
Background: Dendritic cells (DC) are professional antigen-presenting cells in the immune system. They patrol the blood as circulating dendritic cell precursors (DCP). Decreased blood DCP count has been shown to be related to atherosclerotic plaque burden. Since chronic kidney disease (CKD) is associated with chronic inflammation and increased cardiovascular risk, the aim of our study was to investigate a potential effect of CKD on circulating DCP numbers especially in patients with a history of cardiovascular disease.
Methods: The number of circulating myeloid (mDCP), plasmacytoid (pDCP), and total DCP (tDCP) was analysed by flow cytometry in 245 patients with CKD stage 3 (with and without known cardiovascular events) and 85 coronary healthy controls. In addition, data were compared with a historical group of 130 patients with known coronary artery disease (CAD).
Results: Compared to controls, patients with CKD 3 revealed a significant decrease in circulating mDCP (-29%), pDCP (-43%), and tDCP (-38%) (P < 0.001, respectively). Compared with CAD-patients, the decrease in circulating DCP in CKD was comparable or even more pronounced indicating a potential role for DCP in cardiovascular risk potentiation due to CKD.
Conclusions: Based on previous findings in CAD, the marked decrease of DCP in CKD implicates a potential role for DCP as a mediator of cardiovascular disease. Whether DCP in CKD may act as new cardiovascular biomarkers needs to be established in future prospective trials.
Figures
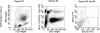
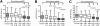

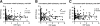
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

