Targeting interleukin-13 with tralokinumab attenuates lung fibrosis and epithelial damage in a humanized SCID idiopathic pulmonary fibrosis model
- PMID: 24325475
- PMCID: PMC4068948
- DOI: 10.1165/rcmb.2013-0342OC
Targeting interleukin-13 with tralokinumab attenuates lung fibrosis and epithelial damage in a humanized SCID idiopathic pulmonary fibrosis model
Abstract
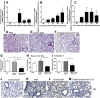
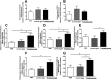
The aberrant fibrotic and repair responses in the lung are major hallmarks of idiopathic pulmonary fibrosis (IPF). Numerous antifibrotic strategies have been used in the clinic with limited success, raising the possibility that an effective therapeutic strategy in this disease must inhibit fibrosis and promote appropriate lung repair mechanisms. IL-13 represents an attractive target in IPF, but its disease association and mechanism of action remains unknown. In the present study, an overexpression of IL-13 and IL-13 pathway markers was associated with IPF, particularly a rapidly progressive form of this disease. Targeting IL-13 in a humanized experimental model of pulmonary fibrosis using tralokinumab (CAT354) was found to therapeutically block aberrant lung remodeling in this model. However, targeting IL-13 was also found to promote lung repair and to restore epithelial integrity. Thus, targeting IL-13 inhibits fibrotic processes and enhances repair processes in the lung.
Figures




Similar articles
-
Modeling Idiopathic Pulmonary Fibrosis in Humanized Severe Combined Immunodeficient Mice.Am J Pathol. 2018 Apr;188(4):891-903. doi: 10.1016/j.ajpath.2017.12.020. Epub 2018 Feb 17. Am J Pathol. 2018. PMID: 29378172 Free PMC article.
-
Targeting of TAM Receptors Ameliorates Fibrotic Mechanisms in Idiopathic Pulmonary Fibrosis.Am J Respir Crit Care Med. 2018 Jun 1;197(11):1443-1456. doi: 10.1164/rccm.201707-1519OC. Am J Respir Crit Care Med. 2018. PMID: 29634284 Free PMC article.
-
DNA-PKcs modulates progenitor cell proliferation and fibroblast senescence in idiopathic pulmonary fibrosis.BMC Pulm Med. 2019 Aug 29;19(1):165. doi: 10.1186/s12890-019-0922-7. BMC Pulm Med. 2019. PMID: 31464599 Free PMC article.
-
MicroRNAs in idiopathic pulmonary fibrosis.Transl Res. 2011 Apr;157(4):191-9. doi: 10.1016/j.trsl.2011.01.012. Epub 2011 Feb 4. Transl Res. 2011. PMID: 21420029 Review.
-
Oxidative stress, extracellular matrix targets, and idiopathic pulmonary fibrosis.Free Radic Biol Med. 2010 Sep 1;49(5):707-17. doi: 10.1016/j.freeradbiomed.2010.04.036. Epub 2010 May 7. Free Radic Biol Med. 2010. PMID: 20452419 Review.
Cited by
-
Resolution of organ fibrosis.J Clin Invest. 2018 Jan 2;128(1):97-107. doi: 10.1172/JCI93563. Epub 2018 Jan 2. J Clin Invest. 2018. PMID: 29293097 Free PMC article. Review.
-
Targeting IL-13 and IL-4 in Asthma: Therapeutic Implications on Airway Remodeling in Severe Asthma.Clin Rev Allergy Immunol. 2025 Apr 21;68(1):44. doi: 10.1007/s12016-025-09045-2. Clin Rev Allergy Immunol. 2025. PMID: 40257546 Free PMC article. Review.
-
Nutraceuticals as potential therapeutics for vesicant-induced pulmonary fibrosis.Ann N Y Acad Sci. 2020 Nov;1480(1):5-13. doi: 10.1111/nyas.14442. Epub 2020 Jul 29. Ann N Y Acad Sci. 2020. PMID: 32725637 Free PMC article. Review.
-
Interleukin-13 disrupts type 2 pneumocyte stem cell activity.JCI Insight. 2020 Jan 16;5(1):e131232. doi: 10.1172/jci.insight.131232. JCI Insight. 2020. PMID: 31941839 Free PMC article.
-
Interleukin-13 Activates Distinct Cellular Pathways Leading to Ductular Reaction, Steatosis, and Fibrosis.Immunity. 2016 Jul 19;45(1):145-58. doi: 10.1016/j.immuni.2016.06.009. Epub 2016 Jul 12. Immunity. 2016. PMID: 27421703 Free PMC article.
References
-
- Kuhn C, III, Boldt J, King TE, Jr, Crouch E, Vartio T, McDonald JA. An immunohistochemical study of architectural remodeling and connective tissue synthesis in pulmonary fibrosis. Am Rev Respir Dis. 1989;140:1693–1703. - PubMed
-
- American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society International multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2002;165:277–304. - PubMed
-
- Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med. 2001;345:517–525. - PubMed
-
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. - PubMed
-
- Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132:1311–1321. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

