Ascorbic acid enhances adipogenesis of 3T3-L1 murine preadipocyte through differential expression of collagens
- PMID: 24325571
- PMCID: PMC3874642
- DOI: 10.1186/1476-511X-12-182
Ascorbic acid enhances adipogenesis of 3T3-L1 murine preadipocyte through differential expression of collagens
Abstract
Background: Adipogenesis from preadipocytes into mature adipocyte is precisely coordinated by transcription factors such as CCAAT-enhancer-binding proteins (C/EBPs) and peroxisome proliferator-activated receptor γ (PPARγ), cytokines, and hormones, which is accompanied by extracellular matrix remodeling. Besides anti-oxidant activity, ascorbic acid (ASC) is participating in collagen biosynthesis and increase production and processing of collagens. Moreover, several studies demonstrated that ASC enhanced differentiation from preadipocytes into mature adipocytes.
Methods: The adipogenic effect of ascorbic acid was evaluated in chemical induced 3T3-L1 by Oil Red O staining. This effect was elucidated by immunoblotting which detected the expression level of collagens and transcription factors in adipogenesis. The immunocytochemical determination of type I collagen was performed in 3T3-L1 adipocyte to show the change of extracellular matrix during adipogenesis.
Results: In this study, Oil Red O staining in 3T3-L1 preadipocytes was increased dose-dependently by addition of ASC. These ASC-treated adipocytes increased collagen processing of α1(I) and α1(V) and expressed α1(VI) and α2(VI) collagens differentially. ASC also stimulated expression of C/EBPα and PPARγ, which is preceded by collagen enhancement. In addition, inhibition of ASC activity by ethyl-3,4-dihydroxybenzoate showed reduction of lipid accumulation by removal of large lipid droplets, not by inhibition of lipid production. This observation went with loss of α1(I) deposition on adipocyte surface, increase of α1(V) and α2(VI) collagens and decrease of C/EBPs.
Conclusion: Our findings imply that various actions of ASC on adipogenesis through differential collagen expression may provide diverse applications of ASC to adipose tissue technology.
Figures
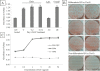


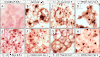
Similar articles
-
Suppression of adipocyte differentiation and lipid accumulation by stearidonic acid (SDA) in 3T3-L1 cells.Lipids Health Dis. 2017 Sep 25;16(1):181. doi: 10.1186/s12944-017-0574-7. Lipids Health Dis. 2017. PMID: 28946872 Free PMC article.
-
Vanadium(IV)-chlorodipicolinate inhibits 3T3-L1 preadipocyte adipogenesis by activating LKB1/AMPK signaling pathway.J Inorg Biochem. 2016 Sep;162:1-8. doi: 10.1016/j.jinorgbio.2016.06.013. Epub 2016 Jun 5. J Inorg Biochem. 2016. PMID: 27318173
-
Tetrandrine has anti-adipogenic effect on 3T3-L1 preadipocytes through the reduced expression and/or phosphorylation levels of C/EBP-α, PPAR-γ, FAS, perilipin A, and STAT-3.Biochem Biophys Res Commun. 2016 Aug 5;476(4):481-486. doi: 10.1016/j.bbrc.2016.05.150. Epub 2016 May 28. Biochem Biophys Res Commun. 2016. PMID: 27246736
-
Vitamin A: A Key Inhibitor of Adipocyte Differentiation.PPAR Res. 2023 Feb 1;2023:7405954. doi: 10.1155/2023/7405954. eCollection 2023. PPAR Res. 2023. PMID: 36776154 Free PMC article. Review.
-
Collagens Regulating Adipose Tissue Formation and Functions.Biomedicines. 2023 May 10;11(5):1412. doi: 10.3390/biomedicines11051412. Biomedicines. 2023. PMID: 37239083 Free PMC article. Review.
Cited by
-
Brown-like adipose progenitors derived from human induced pluripotent stem cells: Identification of critical pathways governing their adipogenic capacity.Sci Rep. 2016 Aug 31;6:32490. doi: 10.1038/srep32490. Sci Rep. 2016. PMID: 27577850 Free PMC article.
-
Ascorbic acid promotes 3T3-L1 cells adipogenesis by attenuating ERK signaling to upregulate the collagen VI.Nutr Metab (Lond). 2017 Dec 28;14:79. doi: 10.1186/s12986-017-0234-y. eCollection 2017. Nutr Metab (Lond). 2017. PMID: 29299041 Free PMC article.
-
Integrative Analysis of miRNAs Involved in Fat Deposition in Different Pig Breeds.Genes (Basel). 2022 Dec 28;14(1):94. doi: 10.3390/genes14010094. Genes (Basel). 2022. PMID: 36672834 Free PMC article.
-
Aggregating in vitro-grown adipocytes to produce macroscale cell-cultured fat tissue with tunable lipid compositions for food applications.Elife. 2023 Apr 4;12:e82120. doi: 10.7554/eLife.82120. Elife. 2023. PMID: 37014056 Free PMC article.
-
Chemical and sensory analyses of cultivated pork fat tissue as a flavor enhancer for meat alternatives.Sci Rep. 2024 Jul 31;14(1):17643. doi: 10.1038/s41598-024-68247-4. Sci Rep. 2024. PMID: 39085314 Free PMC article.
References
-
- Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78(3):783–809. - PubMed
-
- Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7(12):885–896. - PubMed
-
- Weiner FR, Shah A, Smith PJ, Rubin CS, Zern MA. Regulation of collagen gene expression in 3T3-L1 cells. Effects of adipocyte differentiation and tumor necrosis factor alpha. Biochemistry. 1989;28(9):4094–4099. - PubMed
-
- Aratani Y, Kitagawa Y. Enhanced synthesis and secretion of type IV collagen and entactin during adipose conversion of 3T3-L1 cells and production of unorthodox laminin complex. J Biol Chem. 1988;263(31):16163–16169. - PubMed
-
- Yi T, Choi HM, Park RW, Sohn KY, Kim IS. Transcriptional repression of type I procollagen genes during adipocyte differentiation. Exp Mol Med. 2001;33(4):269–275. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

