Tumor necrosis factor-α accelerates the resolution of established pulmonary fibrosis in mice by targeting profibrotic lung macrophages
- PMID: 24325577
- PMCID: PMC4068926
- DOI: 10.1165/rcmb.2013-0386OC
Tumor necrosis factor-α accelerates the resolution of established pulmonary fibrosis in mice by targeting profibrotic lung macrophages
Abstract
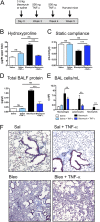
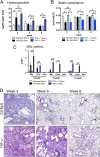
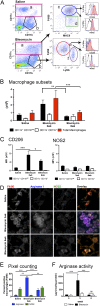
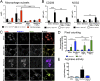
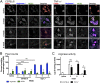
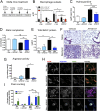
Idiopathic pulmonary fibrosis (IPF) is a relentless, fibrotic parenchymal lung disease in which alternatively programmed macrophages produce profibrotic molecules that promote myofibroblast survival and collagen synthesis. Effective therapies to treat patients with IPF are lacking, and conventional therapy may be harmful. We tested the hypothesis that therapeutic lung delivery of the proinflammatory cytokine tumor necrosis factor (TNF)-α into wild-type fibrotic mice would reduce the profibrotic milieu and accelerate the resolution of established pulmonary fibrosis. Fibrosis was assessed in bleomycin-instilled wild-type and TNF-α(-/-) mice by measuring hydroxyproline levels, static compliance, and Masson's trichrome staining. Macrophage infiltration and programming status was assessed by flow cytometry of enzymatically digested lung and in situ immunostaining. Pulmonary delivery of TNF-α to wild-type mice with established pulmonary fibrosis was found to reduce their fibrotic burden, to improve lung function and architecture, and to reduce the number and programming status of profibrotic alternatively programmed macrophages. In contrast, fibrosis and alternative macrophage programming were prolonged in bleomycin-instilled TNF-α(-/-) mice. To address the role of the reduced numbers of alternatively programmed macrophages in the TNF-α-induced resolution of established pulmonary fibrosis, we conditionally depleted macrophages in MAFIA (MAcrophage Fas-Induced Apoptosis) mice. Conditional macrophage depletion phenocopied the resolution of established pulmonary fibrosis observed after therapeutic TNF-α delivery. Taken together, our results show for the first time that TNF-α is involved in the resolution of established pulmonary fibrosis via a mechanism involving reduced numbers and programming status of profibrotic macrophages. We speculate that pulmonary delivery of TNF-α or augmenting its signaling pathway represent a novel therapeutic strategy to resolve established pulmonary fibrosis.
Figures

References
-
- American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161:646–664. - PubMed
-
- Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–816. - PubMed
-
- Myers JL, Katzenstein AL. Epithelial necrosis and alveolar collapse in the pathogenesis of usual interstitial pneumonia. Chest. 1988;94:1309–1311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL068628/HL/NHLBI NIH HHS/United States
- R01 HL088138/HL/NHLBI NIH HHS/United States
- ES010859/ES/NIEHS NIH HHS/United States
- R01 AI058228/AI/NIAID NIH HHS/United States
- HL090669/HL/NHLBI NIH HHS/United States
- HL034303/HL/NHLBI NIH HHS/United States
- HL088138/HL/NHLBI NIH HHS/United States
- F32 HL095274/HL/NHLBI NIH HHS/United States
- R56 AI058228/AI/NIAID NIH HHS/United States
- AI058228/AI/NIAID NIH HHS/United States
- HL081151/HL/NHLBI NIH HHS/United States
- R01 HL114795/HL/NHLBI NIH HHS/United States
- R01 HL114754/HL/NHLBI NIH HHS/United States
- R01 ES010859/ES/NIEHS NIH HHS/United States
- R01 HL109517/HL/NHLBI NIH HHS/United States
- HL114754/HL/NHLBI NIH HHS/United States
- R01 HL081151/HL/NHLBI NIH HHS/United States
- R01 HL090669/HL/NHLBI NIH HHS/United States
- P01 HL034303/HL/NHLBI NIH HHS/United States
- R01 HL068628/HL/NHLBI NIH HHS/United States
- HL109517/HL/NHLBI NIH HHS/United States
- R01 HL110344/HL/NHLBI NIH HHS/United States
- F32HL095274/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

