Hepatitis B virus surface antigen seroconversion in HIV-infected individual after pegylated interferon-alpha treatment: a case report
- PMID: 24325818
- PMCID: PMC4029789
- DOI: 10.1186/1678-9199-19-31
Hepatitis B virus surface antigen seroconversion in HIV-infected individual after pegylated interferon-alpha treatment: a case report
Abstract
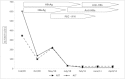
Hepatitis B virus (HBV) infects from 6 to 14% of HIV-infected individuals. Concurrent HIV/HBV infection occurs due to the overlapping routes of transmission, particularly sexual and parenteral. HIV-infected patients that have acute hepatitis B have six times greater risk of developing chronic hepatitis B, with higher viral replication, rapid progression to end-stage liver disease and shorter survival. The coinfection is also associated with poor response to hepatitis B treatment with interferon-alpha and increased liver toxicity to the antiretroviral therapy. Herein, we describe the case of a 35-year-old man who engages in sex with men and presented with newly diagnosed HIV-1, serological markers for acute hepatitis B and progression to chronic hepatitis B infection (HBsAg+ > 6 months, high alanine aminotransferase levels and moderate hepatitis as indicated by liver biopsy). Lacking indication of antiretroviral treatment (CD4 768 cells/mm3), he was treated with pegylated-interferon alpha2b (1.5 mg/kg/week) by subcutaneous injection for 48 weeks. Twelve weeks after treatment, the patient presented HBeAg seroconversion to anti-HBe. At the end of 48 weeks, he presented HBsAg seroconversion to anti-HBs. One year after treatment, the patient maintained sustained virological response (undetectable HBV-DNA). The initiation of antiretroviral therapy with nucleosides and nucleotides is recommended earlier for coinfected individuals. However, this report emphasizes that pegylated interferon remains an important therapeutic strategy to be considered for selected patients, in whom the initiation of HAART may be delayed.
Figures
References
-
- World Health Organization. Global Hepatitis Programme. Geneva: WHO; 2012.
-
- Health W. Organization: Progress Report, Global HIV/AIDS Response. Geneva: WHO; 2011. p. 2011. (Epidemic Update and Health Sector Progress towards Universal Access).
-
- Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;19(1 Suppl):S6–S9. - PubMed
-
- Brasil. Ministério da Saúde. Coordenação Nacional de DST/AIDS. Brasília: Recomendações para Terapia anti–retroviral em adultos infectados pelo HIV – 2007/2008; 2008.
-
- Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Ano II nº 01. Brasília: Departamento de DST, Aids e Hepatites Virais: Boletim epidemiológico hepatites virais; 2011.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials