Mutagenesis and homologous recombination in Drosophila cell lines using CRISPR/Cas9
- PMID: 24326186
- PMCID: PMC3892159
- DOI: 10.1242/bio.20137120
Mutagenesis and homologous recombination in Drosophila cell lines using CRISPR/Cas9
Abstract
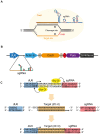
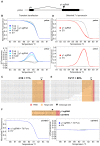
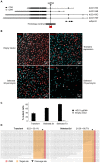
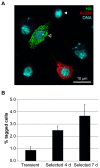
We have applied the CRISPR/Cas9 system to Drosophila S2 cells to generate targeted genetic mutations in more than 85% of alleles. By targeting a constitutive exon of the AGO1 gene, we demonstrate homozygous mutation in up to 82% of cells, thereby allowing the study of genetic knockouts in a Drosophila cell line for the first time. We have shown that homologous gene targeting is possible at 1-4% efficiency using this system, allowing for the construction of defined insertions and deletions. We demonstrate that a 1 kb homology arm length is optimal for integration by homologous gene targeting, and demonstrate its efficacy by tagging the endogenous AGO1 protein. This technology enables controlled genetic manipulation in Drosophila cell lines, and its simplicity offers the opportunity to study cellular phenotypes genome-wide.
Keywords: CRISPR; Cas9; Drosophila S2 cells; Gene targeting; Genome engineering; Homologous recombination.
Conflict of interest statement
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

