Innate antiviral immune signaling, viral evasion and modulation by HIV-1
- PMID: 24326250
- PMCID: PMC7425209
- DOI: 10.1016/j.jmb.2013.12.003
Innate antiviral immune signaling, viral evasion and modulation by HIV-1
Abstract
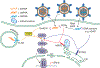
The intracellular innate antiviral response in human cells is an essential component of immunity against virus infection. As obligate intracellular parasites, all viruses must evade the actions of the host cell's innate immune response in order to replicate and persist. Innate immunity is induced when pathogen recognition receptors of the host cell sense viral products including nucleic acid as "non-self". This process induces downstream signaling through adaptor proteins to activate latent transcription factors that drive the expression of genes encoding antiviral and immune modulatory effector proteins that restrict virus replication and regulate adaptive immunity. The interferon regulatory factors (IRFs) are transcription factors that play major roles in innate immunity. In particular, IRF3 is activated in response to infection by a range of viruses including RNA viruses, DNA viruses and retroviruses. Among these viruses, human immunodeficiency virus type 1 (HIV-1) remains a major global health problem mediating chronic infection in millions of people wherein recent studies show that viral persistence is linked with the ability of the virus to dysregulate and evade the innate immune response. In this review, we discuss viral pathogen sensing, innate immune signaling pathways and effectors that respond to viral infection, the role of IRF3 in these processes and how it is regulated by pathogenic viruses. We present a contemporary overview of the interplay between HIV-1 and innate immunity, with a focus on understanding how innate immune control impacts infection outcome and disease.
Keywords: HIV; ISG; innate immunity; interferon; virus.
Copyright © 2013. Published by Elsevier Ltd.
Figures
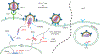

References
-
- Stetson DB, Medzhitov R. Type I interferons in host defense. Immunity 2006;25:373–81. - PubMed
-
- Medzhitov R. Approaching the asymptote: 20 years later. Immunity 2009;30:766–75. - PubMed
-
- Janeway CA. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harbor Symp Quant Biol 1989;54:1–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

