Brain metastasis in renal cancer patients: metastatic pattern, tumour-associated macrophages and chemokine/chemoreceptor expression
- PMID: 24327013
- PMCID: PMC3915122
- DOI: 10.1038/bjc.2013.755
Brain metastasis in renal cancer patients: metastatic pattern, tumour-associated macrophages and chemokine/chemoreceptor expression
Abstract
Background: The mechanisms of brain metastasis in renal cell cancer (RCC) patients are poorly understood. Chemokine and chemokine receptor expression may contribute to the predilection of RCC for brain metastasis by recruitment of monocytes/macrophages and by control or induction of vascular permeability of the blood-brain barrier.
Methods: Frequency and patterns of brain metastasis were determined in 246 patients with metastatic RCC at autopsy. Expression of CXCR4, CCL7 (MCP-3), CCR2 and CD68(+) tumour-associated macrophages (TAMs) were analysed in a separate series of 333 primary RCC and in 48 brain metastases using immunohistochemistry.
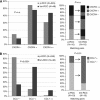
Results: Fifteen percent of 246 patients with metastasising RCC had brain metastasis. High CXCR4 expression levels were found in primary RCC and brain metastases (85.7% and 91.7%, respectively). CCR2 (52.1%) and CCL7 expression (75%) in cancer cells of brain metastases was more frequent compared with primary tumours (15.5% and 16.7%, respectively; P<0.0001 each). The density of CD68(+) TAMs was similar in primary RCC and brain metastases. However, TAMs were more frequently CCR2-positive in brain metastases than in primary RCC (P<0.001).
Conclusion: Our data demonstrate that the monocyte-specific chemokine CCL7 and its receptor CCR2 are expressed in tumour cells of RCC. We conclude that monocyte recruitment by CCR2 contributes to brain metastasis of RCC.
Figures




References
-
- Andre F, Cabioglu N, Assi H, Sabourin JC, Delaloge S, Sahin A, Broglio K, Spano JP, Combadiere C, Bucana C, Soria JC, Cristofanilli M. Expression of chemokine receptors predicts the site of metastatic relapse in patients with axillary node positive primary breast cancer. Ann Oncol. 2006;17 (6:945–951. - PubMed
-
- Bianchi M, Sun M, Jeldres C, Shariat SF, Trinh QD, Briganti A, Tian Z, Schmitges J, Graefen M, Perrotte P, Menon M, Montorsi F, Karakiewicz PI. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol. 2012;23 (4:973–980. - PubMed
-
- Bubendorf L, Schopfer A, Wagner U, Sauter G, Moch H, Willi N, Gasser TC, Mihatsch MJ. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31 (5:578–583. - PubMed
-
- Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30 (7:1073–1081. - PubMed
-
- Culine S, Bekradda M, Kramar A, Rey A, Escudier B, Droz JP. Prognostic factors for survival in patients with brain metastases from renal cell carcinoma. Cancer. 1998;83 (12:2548–2553. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

