Phase I trial of a recombinant yeast-CEA vaccine (GI-6207) in adults with metastatic CEA-expressing carcinoma
- PMID: 24327292
- PMCID: PMC3944709
- DOI: 10.1007/s00262-013-1505-8
Phase I trial of a recombinant yeast-CEA vaccine (GI-6207) in adults with metastatic CEA-expressing carcinoma
Abstract
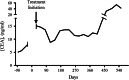
Yeast-CEA (GI-6207) is a therapeutic cancer vaccine genetically modified to express recombinant carcinoembryonic antigen (CEA) protein, using heat-killed yeast (Saccharomyces cerevisiae) as a vector. In preclinical studies, yeast-CEA induced a strong immune response to CEA and antitumor responses. Patients received subcutaneous vaccines every 2 weeks for 3 months and then monthly. Patients were enrolled at 3 sequential dose levels: 4, 16, and 40 yeast units (10(7) yeast particles/unit). Eligible patients were required to have serum CEA > 5 ng/mL or > 20 % CEA(+) tumor block, ECOG PS 0-2, and no history of autoimmunity. Restaging scans were performed at 3 months and then bimonthly. Peripheral blood was collected for the analysis of immune response (e.g., by ELISPOT assay). Twenty-five patients with metastatic CEA-expressing carcinomas were enrolled. Median patient age was 52 (range 39-81). A total of 135 vaccines were administered. The vaccine was well tolerated, and the most common adverse event was grade 1/2 injection-site reaction. Five patients had stable disease beyond 3 months (range 3.5-18 months), and each had CEA stabilization while on-study. Some patients showed evidence post-vaccination of increases in antigen-specific CD8(+) T cells and CD4(+) T lymphocytes and decreases in regulatory T cells. Of note, a patient with medullary thyroid cancer had substantial T cell responses and a vigorous inflammatory reaction at sites of metastatic disease. Yeast-CEA vaccination had minimal toxicity and induced some antigen-specific T cell responses and CEA stabilization in a heterogeneous, heavily pre-treated patient population. Further studies are required to determine the clinical benefit of yeast-CEA vaccination.
Conflict of interest statement
The authors have no conflicts of interest.
Figures


References
-
- Guadagni F, Roselli M, Cosimelli M, Spila A, Cavaliere F, Arcuri R, D’Alessandro R, Fracasso PL, Casale V, Vecchione A, Casciani CU, Greiner JW, Schlom J. Quantitative analysis of CEA expression in colorectal adenocarcinoma and serum: lack of correlation. Int J Cancer. 1997;72(6):949–954. doi: 10.1002/(SICI)1097-0215(19970917)72:6<949::AID-IJC5>3.0.CO;2-P. - DOI - PubMed
-
- Kass ES, Greiner JW, Kantor JA, Tsang KY, Guadagni F, Chen Z, Clark B, De Pascalis R, Schlom J, Van Waes C. Carcinoembryonic antigen as a target for specific antitumor immunotherapy of head and neck cancer. Cancer Res. 2002;62(17):5049–5057. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

